Derive a balanced equation for the reaction occurring in the cell: (a) If E cell = 1.21
Question:
Derive a balanced equation for the reaction occurring in the cell:
![]()
(a) If E°cell = 1.21 V, calculate ΔrG° and the equilibrium constant for the reaction.
(b) Use the Nernst equation to determine the potential for the cell:
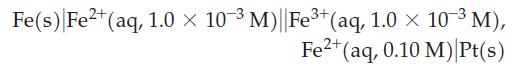
(c) In light of (a) and (b), what is the likelihood of being able to observe the disproportionation of Fe2+ into Fe3+ and Fe under standard conditions?
Transcribed Image Text:
2+ 3+ Fe(s) Fe²+ (aq)| Fe³+ (aq), Fe²+ (aq) Pt(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
The balanced equation for the reaction occurring in the cell is Fes 2 Fe3 3 Fe2 This i...View the full answer

Answered By

Ali Khawaja
my expertise are as follows: financial accounting : - journal entries - financial statements including balance sheet, profit & loss account, cash flow statement & statement of changes in equity -consolidated statement of financial position. -ratio analysis -depreciation methods -accounting concepts -understanding and application of all international financial reporting standards (ifrs) -international accounting standards (ias) -etc business analysis : -business strategy -strategic choices -business processes -e-business -e-marketing -project management -finance -hrm financial management : -project appraisal -capital budgeting -net present value (npv) -internal rate of return (irr) -net present value(npv) -payback period -strategic position -strategic choices -information technology -project management -finance -human resource management auditing: -internal audit -external audit -substantive procedures -analytic procedures -designing and assessment of internal controls -developing the flow charts & data flow diagrams -audit reports -engagement letter -materiality economics: -micro -macro -game theory -econometric -mathematical application in economics -empirical macroeconomics -international trade -international political economy -monetary theory and policy -public economics ,business law, and all regarding commerce
4.00+
1+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Read the statement below and answer all five questions that follow: The procurement professional task is to reduce the cost of a product, material or service through supplier relationship development...
-
What would you expect to happen when chlorine gas, Cl2, at 1 atm pressure is bubbled into a solution containing 1.0 M F and 1.0 M Br at 25oC? Write a balanced equation for the reaction that occurs.
-
Bromine is obtained from brine wells. The process involves treating water containing bromide ion with Cl 2 and extracting the Br 2 from the solution using an organic solvent. Write a balanced...
-
In the proposal, the contractor estimated that 50 units of a specialty part are required. Each unit costs $300. There is a minimum buy requirement of 100 units. You contact the vendor and confirm the...
-
How do legal factors affect international sales management?
-
In the following summary of data for a payroll period, some amounts have been intentionally omitted: a. Calculate the amounts omitted in lines (1), (3), (7), and (11). b. Record the entry for the...
-
Redesign the fractionator of Example 6.8 for a reflux ratio that is twice the minimum. Determine the diameter of the tower, the height of packing in the stripping and rectifying sections, and the...
-
A manufacturer is considering alternatives for building new plants, to be located closer to three of its primary customers with whom it intends to develop long-term, sole-supplier relationships. The...
-
Solve problems involving parallelograms, trapezoids and kites. Write the correct answer in the answer sheet. 1. A cross section of a water trough is in the shape of a trapezoid with bases measuring...
-
For the alkaline Leclanch cell. (a) write the overall cell reaction. (b) Determine E cell for that cell reaction. Figure 19.14 The Leclanch (Dry) Cell The most common form of voltaic cell is the...
-
For the voltaic cell, (a) what isE cell initially? (b) As the cell operates, willE cell increase, decrease, or remain constant with time? Explain. (c) What will beE cell when [Ag + ] has increased to...
-
What are general capital assets? How are they reported in the fund and government-wide financial statements?
-
If you had to pick one mobile affordance that is the most important , which one would it be and why? In your answer, make sure to explain that affordance (based on the reading and lecture) and...
-
Figure 1: Cash schedule for a whole life plan: guaranteed and projected 3. Basic Plan - Illustration Summary SURRENDER VALUE DEATH BENEFIT Guaranteed Non-Guaranteed Non-Guaranteed End of Policy Year...
-
Using the "enterprise method", calculate the value of the entire business. You can assume a 35% tax rate. Hint: you may not required to use all the given items. EBIT Net Income Depreciation Changes...
-
2. Citibikes were introduced in New York City in 2013. At first, they were only south of 59th street in Manhattan, now they are available throughout Man- hattan, and many parts of Brooklyn, Queens,...
-
You purchased 100 shares of stock for a share price of $16.84. You sold the stock two years later for a share price of $18.78. You also received total dividend payments of $0.77 per share. What was...
-
On the basis of the information provided, under IFRSs, is goodwill associated with the Spanish operations impaired as of December 31, 2010? If so, determine the impairment loss and the new carrying...
-
A Firm intends to invest some capital for a period of 15 years; the Firm's Management considers three Options, each consisting of purchasing a machinery of a specific brand, different for each...
-
Based on the data presented in Exercise 25-15, assume that MyPhone Inc. uses the product cost concept of applying the cost-plus approach to product pricing. (a) Determine the total manufacturing...
-
Based on the data presented in Exercise 25-15, assume that MyPhone Inc. uses the variable cost concept of applying the cost-plus approach to product pricing. (a) Determine the variable costs and the...
-
Toyota Motor Corporation uses target costing. Assume that Toyota marketing personnel estimate that the competitive selling price for the Camry in the upcoming model year will need to be $22,000....
-
Aerospace contractor has four employees whose combined salaries through the end of this year are $250,000. If the contractor expect to give an average raise of 5% each year, calculate the present...
-
With the aid of a diagram, explain Project Risk Management process in the context of a new Solar Plant which Chinhoyi Municipality intends to establish to address its power needs.
-
Do moral and ethic values cover everything while law does not?

Study smarter with the SolutionInn App


