Formamide, used in the manufacture of pharmaceuticals, dyes, and agricultural chemicals, decomposes at high temperatures. If 0.186
Question:
Formamide, used in the manufacture of pharmaceuticals, dyes, and agricultural chemicals, decomposes at high temperatures.
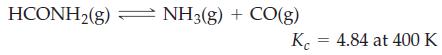
If 0.186 mol HCONH2(g) dissociates in a 2.16 L flask at 400 K, what will be the total pressure at equilibrium?
Transcribed Image Text:
HCONH₂(g) NH3(g) + CO(g) K 4.84 at 400 K
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To find the total pressure at equilibrium for the given reaction of formamide decomposition HCONH2g ...View the full answer

Answered By

Loise Ndungu
I have five years of experience as a writer. As I embark on writing your papers from the prologue to the epilogue, my enthusiasm is driven by the importance of producing a quality product. I put premium product delivery as my top priority, as this is what my clients are seeking and what makes me different from other writers. My goal is to craft a masterpiece each time I embark on a freelance work task! I'm a freelance writer who provides his customers with outstanding and remarkable custom writings on various subjects. Let's work together for perfect grades.
4.90+
78+ Reviews
224+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The compound SbCl5(g) decomposes at high temperatures to gaseous antimony trichloride and chlorine gas. When 89.7 g of SbCl5(g) is placed in a 15.0-L container at 1808C, the SbCl5(g) is 29.2%...
-
The head box process is used in the manufacture of paper to transform the pulp slurry flow into a jet of 2 cm and then spread it onto a mesh belt [22]. To achieve desirable paper quality, the pulp...
-
The horizontal range and the maximum height of a projectile are equal. The angle of projection of the projectiles is: (a) 0 = tan() (b) 0 = tan (4) (c) 0 = tan (2) (d) 0 = 45
-
The drawing shows a frictionless incline and pulley. The two blocks are connected by a wire (mass per unit length = 0.0250 kg/m) and remain stationary. A transverse wave on the wire has a speed of...
-
Levin Corporation has fixed operating costs of $72,000, variable operating costs of $6.75 per unit, and a selling price of $9.75 per unit. a. Calculate the operating breakeven point in units. b....
-
Irwin Schiff is a self-styled tax rebel who has made a career, and substantial profit, out of his tax protest activities. On February 7, Schiff appeared live on CBS News Nightwatch, a late-night...
-
99 percent per month. In 2014, Boling resolved his suit against the gas can manufacturer. Shortly thereafter, Prospect sent Boling a Schedule of Purchases, asserting that Boling owed Prospect...
-
Below are selected T-accounts for the RunnerTech Company. Below are selected T-accounts for the RunnerTech Company. Required: Complete the following journal entries and answer the following...
-
When two electric charges are held a distance r apart, the electrostatic force between them is FE. The distance between the charges is then changed to 1r. (Enter numerical value only) The new...
-
A mixture of 1.00 mol NaHCO 3 (s) and 1.00 mol Na 2 CO 3 (s) is introduced into a 2.50 L flask in which the partial pressure of CO 2 is 2.10 atm and that of H 2 O(g) is 715 mmHg. When equilibrium is...
-
For the following reaction, K c = 2.00 at 1000 C. If a 5.00 L mixture contains 0.145 mol COF 2 , 0.262 mol CO 2 , and 0.074 mol CF 4 at a temperature of 1000 C, (a) Will the mixture be at...
-
Explain that to qualify for the HCISPP you must focus on security management topics and healthcare; this certification requires the candidate to demonstrate knowledge in six specialty domains on its...
-
Amy plans to repay a loan of $360,000 by making payments of $60,000 at the end of each year for 10 years. She replaces the capital by means of a sinking fund and pays interest on the loan at an...
-
A company plans to issue a $1,000 par value, 11-year noncallable bond with a 15% annual coupon, paid semiannually. The company's marginal tax rate is 41%, but there is legislation that is considering...
-
You have obtained the following data: D 0 = $1.63; P 0 = $57; and g = 7.4% (constant). Based on the DCF approach, what is the cost of equity from retained earnings?
-
A project has the following cash flows over the next four years starting right now; -5000; 1500; 1500; 3,608; 4000. The projects cost of capital is 8 percent. What is the projects payback period?
-
Question: Discuss the pros and cons of architectures with a 64-bit word size versus those with a 32-bit word size. Which applications are likely to benefit most? [4 marks] (c) If you were a processor...
-
The trial balance of Brennan Fashion Center contained the following accounts at November 30, the end of the company's fiscal year. BRENNAN FASHION CENTER Trial Balance 30-Nov-02 Adjustment data: 1....
-
What are the principal differences among asset liquidity management, liability management, and balanced liquidity management?
-
Warwick Bottle Company (WBC) manufactures plastic two-liter bottles for the beverage industry. The cost standards per 100 two-liter bottles are as follows: At the beginning of July, WBC management...
-
The following data relate to the direct materials cost for the production of 2,000 automobile a. Determine the price variance, quantity variance, and total direct materials cost variance.b. To whom...
-
I-Time, Inc., produces electronic timepieces. The company uses mini-LCD displays for its products. Each timepiece uses one display. The company produced 550 timepieces during March. However, due to...
-
What are the benefits of employing Secure Socket Layer (SSL) ? Why is the main purpose of using secure socket layer SSL )?
-
Pick a distinct industry: banking, finance, retail, healthcare, national security, etc. Describe the types of sensitive information that an organization in that industry may maintain. Describe...
-
You are considering enrolling in a 2-year long MBA program. You will continue your current employment. If you do, you must pay two payments of $30,000; the first happens today, at the beginning of...

Study smarter with the SolutionInn App


