Question: From the data given in Exercise 72, estimate a value of r S at 298 K for the reaction Exercise 72 Sodium carbonate, an
From the data given in Exercise 72, estimate a value of ΔrS° at 298 K for the reaction
![]()
Exercise 72
Sodium carbonate, an important chemical used in the production of glass, is made from sodium hydrogen carbonate by the reaction
![]()
Data for the temperature variation of K for this reaction are K = 1.66 x 10-5 at 30 °C; 3.90 x 10-4 at 50 °C; 6.27 x 10-3 at 70 °C; and 2.31 x 10-1 at 100 °C.
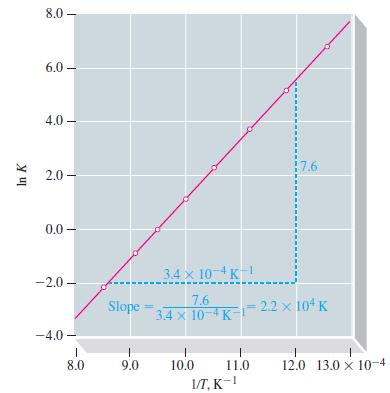
(a) Plot a graph similar to Figure 13-10, and determine ΔrH° for the reaction.
(b) Calculate the temperature at which the total gas pressure above a mixture of NaHCO3(s) and Na2CO3(s) is 2.00 bar.
Figure 13-10
2 NaHCO3(s) Na2CO3(s) + HO(g) + CO2(g)
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


