In the Dow process (Fig. 21-13), the starting material is Mg 2+ in seawater and the final
Question:
In the Dow process (Fig. 21-13), the starting material is Mg2+ in seawater and the final product is Mg metal. This process seems to violate the principle of conservation of charge. Does it? Explain.
Figure 21-13
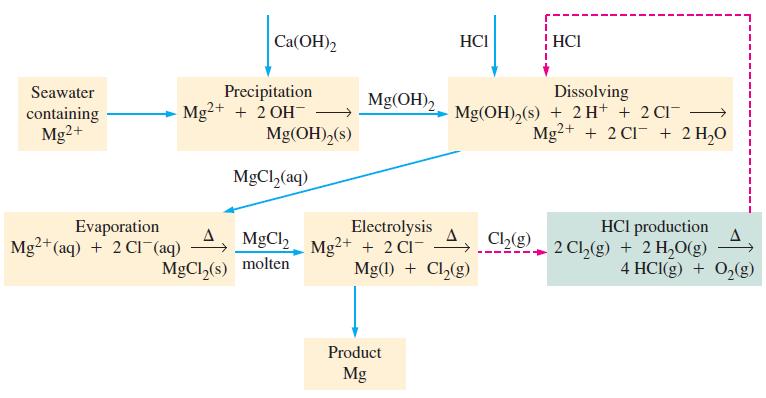
Transcribed Image Text:
Seawater containing Mg2+ Ca(OH)2 Precipitation Mg²+ + 2 OH- Evaporation Mg2+ (aq) + 2 CI (aq) Mg(OH)2 (s) MgCl₂(aq) MgCl, MgCl₂(s) molten Mg(OH)₂ Electrolysis Mg²+ + 2 CI- HCI Product Mg Mg(1) + Cl₂(g) HCI Dissolving Mg(OH)₂(s) + 2 H+ + 2 CI¯ Mg2+ + 2 C1 + 2 H₂O HCI production Chg 2 Ch₂(g) + 2 H₂O(g) Ch₂(g) 1 4 HCl(g) + O₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
No the Dow process does not violate the principle of conservation of charge The process starts with ...View the full answer

Answered By

Saikumar Ramagiri
Financial accounting:- Journal and ledgers, preparation of trail balance and adjusted trail balance Preparation of income statement, retained earning statement and balance sheet Banks reconciliation statements Financial statement analysis Cash flow statement analysis (both direct and indirect methods) All methods of Depreciations Management Accounting:- Ratios Budgeting control Cash budget and production budget Working capital management Receivable management Costing:- Standard and variance costing Marginal costing and decision making Cost-volume-profit analysis Inventory management (LIFO, FIFO) Preparation and estimation of cost sheet Portfolio management:- Calculation of portfolio standard deviation or risk Calculation of portfolio expected returns CAPM, Beta Financial management:- Time value of money Capital budgeting Cost of capital Leverage analysis and capital structure policies Dividend policy Bond value calculations like YTM, current yield etc International finance:- Derivatives Futures and options Swaps and forwards Business problems Finance problems Education (mention all your degrees, year awarded, Institute/University, field(s) of major): Education Qualification Board/Institution/ University Month/Year of Passing % Secured OPTIONALS/ Major ICWAI(inter) ICWAI inter Pursuing Pursuing - M.com(Finance) Osmania University June 2007 65 Finance & Taxation M B A (Finance) Osmania University Dec 2004 66 Finance & Marketing. B.Com Osmania University June 2002 72 Income Tax, Cost & Mgt, Accountancy, Auditing. Intermediate (XII) Board of Intermediate May 1999 58 Mathematics, Accountancy, Economics. S S C (X) S S C Board. May 1997 74 Mathematics, Social Studies, Science. Tutoring experience: • 10 year experience in online trouble shooting problems related to finance/accountancy. • Since 6 Years working with solution inn as a tutor, I have solved thousands of questions, quick and accuracy Skills (optional): Technical Exposure: MS Office, SQL, Tally, Wings, Focus, Programming with C Financial : Portfolio/Financial Management, Ratio Analysis, Capital Budgeting Stock Valuation & Dividend Policy, Bond Valuations Individual Skills : Proactive Nature, Self Motivative, Clear thought process, Quick problem solving skills, flexible to complex situations. Achievements : 1. I have received an Award certificate from Local Area MLA for the cause of getting 100% marks in Accountancy during my Graduation. 2. I have received a GOLD MEDAL/Scholarship from Home Minister in my MBA for being the “Top Rank student “ of management institute. 3. I received numerous complements and extra pay from various students for trouble shooting their online problems. Other interests/Hobbies (optional): ? Web Surfing ? Sports ? Watching Comics, News channels ? Miniature Collection ? Exploring hidden facts ? Solving riddles and puzzles
4.80+
391+ Reviews
552+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Q1. How worried are clients and stakeholders in day-to- day product improvement? 2. the industrial corporation Case for Agility "The struggle is not always to the most powerful, nor the race to the...
-
When o-chlorotoluene is subjected to the conditions used in the Dow process (i.e., aqueous NaOH at 350oC at high pressure), the products of the reaction are o-cresol and m-cresol. What does this...
-
List three specific parts of the Case Guide, Objectives and Strategy Section (See below) that you had the most difficulty understanding. Describe your current understanding of these parts. Provide...
-
2. National Defense (40 points). There are 11 countries in Europe who get utility from general consumption c, and from European national defense G. The utility of a generic country i is u(ci, G) =...
-
Refer to PE 410. 1. Make the adjusting entry necessary on the companys books on December 31 with respect to this insurance policy 2. Compute the ending balance in the prepaid insurance account....
-
Tyler Company acquired all of Jasmine Companys outstanding stock on January 1, 2009, for $206,000 in cash. Jasmine had a book value of only $140,000 on that date. However, equipment (having an eight...
-
Define a sequence of correlated random numbers \[ s_{k}=\alpha s_{k-1}+(1-\alpha) r_{k} \] where \(r_{k}\) is a unit-variance, uncorrelated, Gaussian pseudorandom number while \(0
-
Rudd Clothiers is a small company that manufactures tall-mens suits. The company has used a standard cost accounting system. In May 2017, 11,250 suits were produced. The following standard and actual...
-
K Use appropriate formulas to find (a) the perimeter and (b) the area of the figure. 8.8 m 5 m 5.2 m 7 m (a) The perimeter is (Type an integer or a decimal.)
-
Without performing detailed calculations, indicate why you would expect each of the following reactions to occur to a significant extent as written. Use data from Appendix D as necessary. (a)...
-
To prevent the air oxidation of aqueous solutions of Sn 2+ to Sn 4+ , metallic tin is sometimes kept in contact with the Sn 2+ (aq). Suggest how this contact helps prevent the oxidation.
-
Data 4.5 on page 228 discusses a test to determine if the mean level of arsenic in chicken meat is above 80 ppb. If a restaurant chain finds significant evidence that the mean arsenic level is above...
-
Consider the case of instant runoff voting when three or more social choices are involved. Each voter has one vote and votes for his or her favorite option. The option with the least votes is...
-
Fifty-four million dollars for a pair of missing pants? A judge in Washington, D.C., made headlines when he filed a \($54\) million lawsuit against his neighborhood dry cleaner because it lost a pair...
-
A woman in New Zealand apparently died from drinking too much Coca-Cola. Her family said she drank about 2.2 gallons of the beverage every day for years. Prior to her death, she had several rotten...
-
On 31 October 2006, in an effort to halt the rapid growth of income trust structures in the Canadian stock market, Canadas Minister of Finance James Flaherty announced that these tax-exempt...
-
Analysts commonly encounter a number of labels related to the cyclical/non-cyclical distinction. For example, non-cyclical industries have sometimes been sorted into defensive (or stable) versus...
-
Most expenditures that have a business purpose and meet the ordinary, necessary, and reasonable requirements are deductible. However, specific rules must be adhered to in determining the...
-
Global.asax is used for: a. declare application variables O b. all other answers are wrong O c. declare global variables O d. handle application events
-
Kopke Company, organized in 2012, has these transactions related to intangible assets in that year: Jan. 2 Purchased a patent (5-year life) $280,000. Apr. 1 Goodwill acquired as a result of purchased...
-
Alliance Atlantis Communications Inc. changed its accounting policy to amortize broadcast rights over the contracted exhibition period, which is based on the estimated useful life of the program....
-
The questions listed below are independent of one another. Instructions Provide a brief answer to each question. (a) Why should a company depreciate its buildings? (b) How can a company have a...
-
A. Define the term Scope Creep and list TWO (2) ways to manage scope creep. B. Explain ANY TWO(2) ways in which a project may be terminated. C. Outline the difference between ANY TWO (2 ) factors...
-
How do Freudian and Jungian approaches to symbolism illuminate the psychoanalytic dimensions of literary texts, uncovering unconscious desires, fears, and complexes encoded within symbolic imagery...
-
Describe some of the potential freight applications of these modes of transportation: truck, freight train, Airline, ocean shipping, river bargetransportation. Give examples where each logistics...

Study smarter with the SolutionInn App


