Question: It is possible to write simple equations to relate pH, pK, and concentrations (c) of various solutions. Three such equations are shown here. (a) Derive
It is possible to write simple equations to relate pH, pK, and concentrations (c) of various solutions. Three such equations are shown here.
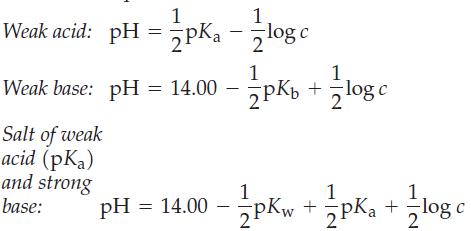
(a) Derive these three equations, and point out the assumptions involved in the derivations.
(b) Use these equations to determine the pH of 0.10 M CH3COOH(aq), 0.10 M NH3(aq), and 0.10 M NaCH3COO(aq).
Verify that the equations give correct results by determining these pH values in the usual way.
1 Weak acid: pH = pK - 1log c 1 Weak base: pH = 14.00 --- pKb 2PK Salt of weak acid (pka) and strong base: +=log c 1 1 pH = 14.00 - 2pKw+ Pka + log c
Step by Step Solution
3.35 Rating (158 Votes )
There are 3 Steps involved in it
Lets derive and discuss the three given equations for weak acids weak bases and salts of weak acids and strong bases After that well use these equatio... View full answer

Get step-by-step solutions from verified subject matter experts


