Magnesiumaluminum alloys are widely used in aircraft construction. One particular alloy contains 70.0% Al and 30.0% Mg,
Question:
Magnesium–aluminum alloys are widely used in aircraft construction. One particular alloy contains 70.0% Al and 30.0% Mg, by mass. How many grams of H2(g) are produced in the reaction of a 0.710 g sample of this alloy with excess HCl(aq)? Balanced chemical equations are given below for the reactions that occur.
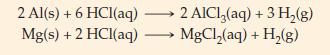
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





