One glucose molecule, C 6 H 12 O 6 (s), is converted to two lactic acid molecules,
Question:
One glucose molecule, C6H12O6(s), is converted to two lactic acid molecules, CH3CH(OH)COOH(s) during glycolysis. Given the combustion reactions of glucose and lactic acid, determine the standard enthalpy for glycolysis.
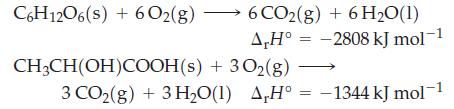
Transcribed Image Text:
C6H12O6(s) + 602(g) 6 CO2(g) + 6H₂O(1) A,H° ΔΗ° CH₂CH(OH)COOH(s) + 3O₂(g) 3CO2(g) +3H,O(1) AH° -2808 kJ mol-1 -1344 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To determine the standard enthalpy for glycolysis using the given information we can use Hesss Law H...View the full answer

Answered By

Benish Ahmad
I'm a professional software engineer. I'm lectutrer at GCUF and I have 3 years of teaching experience. I'm looking forward to getting mostly computer science work including:
Programming fundamentals
Object oriented programming
Data structures
object oriented design and analysis
Database system
Computer networks
Discrete mathematics
Web application
I am expert in different computer languages such as C++, java, JavaScript, Sql, CSS, Python and C#. I'm also have excellent knowledge of essay writing and research. I have worked in other Freelancing website such as Fiverr and Upwork. Now I have finally decided to join the SolutionInn platform to continue with my explicit work of helping dear clients and students to achieve their academic dreams. I deliver plagiarism free work and exceptional projects on time. I am capable of working under high pressure.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
In biological cells that have a plentiful supply of oxygen, glucose is oxidized completely to CO 2 and H 2 O by a process called aerobic oxidation. Muscle cells may be deprived of O 2 during vigorous...
-
Choose a conflict situation you experienced in a work setting. Make sure that your illustration is work-related and not personal. Prepare a formal report, with recommendations you would make, based...
-
Can we use servomotor for position control? Support the answer with necessary details
-
Consider the hydrogen atom, and assume that the proton, instead of being a point- source of the Coulomb field, is uniformly charged sphere of radius R ( < < ao), so that the Coulomb potential is now...
-
Assume that you must make a presentation to the marketing staff explaining the difference between product and period costs. Your supervisor tells you the marketing staff would also like clarification...
-
Based on the case study illustration and the eff ect of changing the annual compensation rate, the annual unit credit for the average participant would decrease by an amount closest to: A . 4,349. B...
-
Taj Mahabub was the founder and CEO of GenAudio, a Colorado-based audio technology company. GenAudio had struggled financially practically since its formation, and Mahabub wished to secure a...
-
A company begins a review of ordering policies [or its continuous review system by checking the current policies for a sample of SKUs. Following are the characteristics of one item. Demand (D) =64...
-
A 2 kg block is attached to a spring with a force constant of 400 N/m. The block is initially at rest and is compressed by 0.5 meters from its equilibrium position. When released, the block undergoes...
-
Using the factory energy cost data in Exhibit 11.11, find the best moving average and exponential smoothing models. Compare their forecasting ability with the regression model developed in the...
-
Use standard enthalpies of formation from Table 7.2 and equation (7.22) to determine the standard enthalpy of reaction in the following reactions. Table 7.2 Eq. 7.22 (a) C3H8(g) + H(g) CH6(g) +...
-
The standard heats of combustion ( r H) of buta-1,3-diene, C 4 H 6 (g); butane, C 4 H 10 (g); and H 2 (g) are -2540.2, -2877.6, and -285.8 kJ mol-1, respectively. Use these data to calculate the heat...
-
Table 12.4 on page 482 showed the calculated sums of the observed frequencies, the expected frequencies, and their differences. Strictly speaking, those sums are not needed. However, they serve as a...
-
Suppose you plan to use multiples to estimate the stock price of Electronic Arts (EA). You observed the following quantities from a list of comparable companies in the same industry P/E Take Two...
-
In the video "Professionalism in Writing," I talk about how creativity relates to professionalism. Can you describe that relationship? Edit View Incort
-
You work for the Chartered Accountancy firm Bevan & Bevan Ltd, which has, for many years, been engaged to perform accounting and corporation tax work. Currently, you have discover that some practices...
-
There are 3 resistors in series: R1 = 37 Ohm, R2 = 24 Ohm, and R3 = 3 Ohm. Determine the equivalent resistance. Show all work and steps: 1. Type the given information and assign a variable for each...
-
Many gas stations give a discount for paying with cash instead of a credit card. William's local gas station gives a discount of $0.05 per gallon. He fills his car with 20 gallons of gas. How much...
-
Refer to the data provided in E4A. In E4A. In chronological order, the inventory, purchases, and sales of a single product for a recent month are as follows. 1. Using the perpetual inventory system,...
-
In the synthesis of the keto acid just given, the dicarboxylic acid decarboxylates in a specific way; it gives Explain. HO rather than HO
-
Distinguish between counterbalancing and non-counterbalancing errors. Give an example of each.
-
Discuss and illustrate how a correction of an error in previously issued financial statements should be handled.
-
Prior to 2010, Heberling Inc. excluded manufacturing overhead costs from work in process and finished goods inventory. These costs have been expensed as incurred. In 2010, the company decided to...
-
Calculate the change in internal energy when 250 grams of ice at 0 C is melted and turned into 250 grams of liquid water at 0 C.
-
Karen goes on a road trip; her car gets 22 miles per gallon (mpg) and gas costs $3.38 per gallon. Let n represent the number of miles Karen has traveled since she started driving. a. Suppose Karen...
-
Your company is reviewing a project with estimated labor costs of $14.68 per unit, estimated raw material costs of $43.18 a unit, and estimated fixed costs of $18,000 a month. Sales are projected at...

Study smarter with the SolutionInn App


