Use standard enthalpies of formation from Table 7.2 and equation (7.22) to determine the standard enthalpy of
Question:
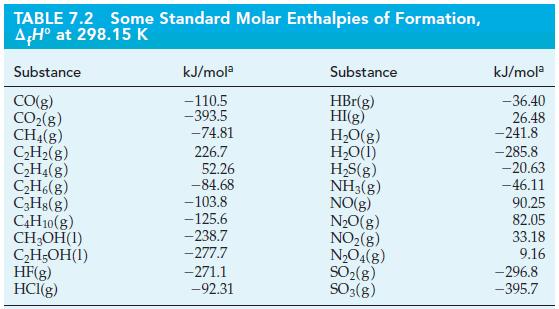
Use standard enthalpies of formation from Table 7.2 and equation (7.22) to determine the standard enthalpy of reaction in the following reactions.
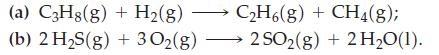
Table 7.2
Eq. 7.22
![A,H [cx AHc + dx AHD + ...]-[ax AHA + bx AHB +...] (7.22) weighted sum of A H values for the products](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/0/4/241654de711110c61699604236540.jpg)
Transcribed Image Text:
(a) C3H8(g) + H₂(g) → C₂H6(g) + CH4(g); (b) 2 H₂S(g) + 30₂(g) →→→2SO₂(g) + 2 H₂O(1).
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a AHTxnAH products AH reactants ...View the full answer

Answered By

Nandana Wijayarathna
I am a highly experienced writer in several areas,
Business management
Information technology
Business administration
Literature
Biology
Environmental science
History
4.50+
161+ Reviews
399+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use standard enthalpies of formation from Tables 7.2 and 7.3 and equation (7.22) to determine the standard enthalpy of reaction in the following reaction. Tables 7.2 Tables 7.3 Eq. 7.22 NH4+ (aq) +...
-
The standard enthalpies of formation of ClO and ClO2 are 101 and 102 kJ/mol, respectively. Using these data and the thermodynamic data in Appendix C, calculate the overall enthalpy change for each...
-
Use standard enthalpies of formation from Table 7.2 to determine r H at 25 C for the following reaction. Table 7.2 2 Cl(g) + 2 HO(1) 4 HCl(g) + O(g) A,H = ?
-
Riffa Football Club is planning to organize a football tournament to raise charity funds. The estimated costs per match Amount paid to Players, Coaches and Referees BHD 1,400; Ground Rent BHD 250;...
-
Refer to Decision Maker, Purchase Manager, in this chapter. Assume that you are the motorcycle manufacturers managerial accountant. The purchasing manager asks you about preparing an estimate of the...
-
Th e current service cost is closest to: A . $14,152. B . $15,758. C . $17,907.
-
The Nielsen family formed their corporation, N. Robert Nielsen, Inc., to conduct farming operations. Morre, Grider & Co. is a certified public accounting firm that has provided accounting, tax, and...
-
Green Pastures is a 400-acre farm on the outskirts of the Kentucky Bluegrass, specializing in the boarding of broodmares and their foals. A recent economic downturn in the thoroughbred industry has...
-
Map this ER diagram to a Relational Model and normalize it if needed. employee EMP_ID INT EMP_Frame VARCHAR(40) EMP_Lname VARCHAR(40) EMP_Sex VARCHAR(1) EMP_Birthdate DATE EMP_Salary INT Indexes...
-
On May 2, 1988, Hannah Weather (Social Security number: 111-22- 3333) acquired residential real estate for $450,000. Of the cost, $100,000 was allocated to the land and $350,000 to the building. On...
-
The standard enthalpy of fermentation of glucose to ethanol is Use the standard enthalpy of combustion for glucose to calculate the enthalpy of combustion for ethanol. C6H12O6(s)- 2 CH3CHOH(1) + 2...
-
One glucose molecule, C 6 H 12 O 6 (s), is converted to two lactic acid molecules, CH 3 CH(OH)COOH(s) during glycolysis. Given the combustion reactions of glucose and lactic acid, determine the...
-
Discuss whether property that is classified as personal use is subject to cost recovery. Discuss the difference between personal property and personal use property. Please give examples.
-
A taxpayer and their family lose their home in a wildfire, and their insurance company puts them up in a hotel for a few weeks, along with an additional stipend for covering food and certain other...
-
If Dave makes this investment, he will use money currently invested in tax free municipal bonds expected to yield 5% for the foreseeable future. His taxable income would be taxed as individual...
-
Question 2: The Town of Rockville has two bonds outstanding. The first bond is a one-year zero coupon bond, the second is a two-year zero-coupon bond. The yield to maturity on the first is 6.0%, and...
-
What would be an example of a "Perfectly Inelastic Collision" in real life? Explain.
-
What percent of total income in US is earned by the top one percent of earners? What are the contributing factors to growing income and wealth inequalities in US?
-
During July 2014, Micanopy, Inc., sold 500 units of its product Empire for $8,000. The following units were available: A sale of 500 units was made after purchase 3. Of the units sold, 200 came from...
-
At the beginning of its fiscal year, Lakeside Inc. leased office space to LTT Corporation under a seven-year operating lease agreement. The contract calls for quarterly rent payments of $25,000 each....
-
Discuss how a change to the LIFO method of inventory valuation is handled when it is impracticable to determine previous LIFO inventory amounts.
-
How should consolidated financial statements be reported this year when statements of individual companies were presented last year?
-
Simms Corp. controlled four domestic subsidiaries and one foreign subsidiary. Prior to the current year, Simms Corp. had excluded the foreign subsidiary from consolidation. During the current year,...
-
Part A An object with mass 2.7 kg is executing simple harmonic motion, attached to a spring with spring constant 330 N/m. When the object is 0.019 m from its equilibrium position, it is moving with a...
-
toyo Manufacturing has 1,000,000 shares of common stock outstanding at a market price of $40.20 a share. Last month, the company paid an annual dividend in the amount of $2.34 per share. The dividend...
-
Solve the homogeneous linear system and use this solution to write down a fundamental matrix (t) Use (t) to compute e At where 1

Study smarter with the SolutionInn App


