Question: Plot the following data first as boiling point versus polarizability, and then as boiling point versus molecular mass. What conclusions can you draw from these
Plot the following data first as boiling point versus polarizability, and then as boiling point versus molecular mass. What conclusions can you draw from these plots?
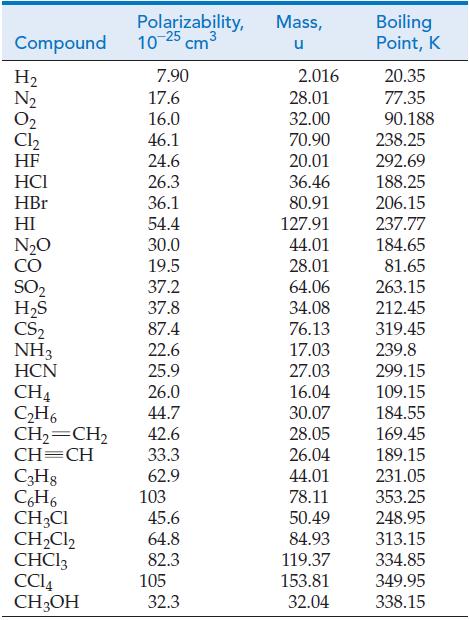
Compound H N Cl HF HCI HBr HI NO CO SO HS CS NH3 HCN CH4 CH6 Polarizability, 10-25 cm C3H8 C6H6 CH,C1 CHCl2 CHC13 CC14 CH3OH 7.90 17.6 16.0 46.1 24.6 26.3 36.1 54.4 30.0 19.5 37.2 37.8 87.4 22.6 25.9 26.0 44.7 CH=CH 42.6 CH=CH 33.3 62.9 103 45.6 64.8 82.3 105 32.3 Mass, u 2.016 28.01 32.00 70.90 20.01 36.46 80.91 127.91 44.01 28.01 64.06 34.08 76.13 17.03 27.03 16.04 30.07 28.05 26.04 44.01 78.11 50.49 84.93 119.37 153.81 32.04 Boiling Point, K 20.35 77.35 90.188 238.25 292.69 188.25 206.15 237.77 184.65 81.65 263.15 212.45 319.45 239.8 299.15 109.15 184.55 169.45 189.15 231.05 353.25 248.95 313.15 334.85 349.95 338.15
Step by Step Solution
3.31 Rating (160 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


