Sodium cyclopentadienide, NaC 5 H 5 , is a common reducing agent in the chemical laboratory, but
Question:
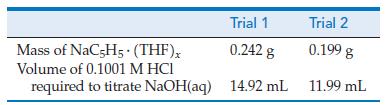
Sodium cyclopentadienide, NaC5H5, is a common reducing agent in the chemical laboratory, but there is a problem in using it: NaC5H5 is contaminated with tetrahydrofuran (THF), C4H8O, a solvent used in its preparation. The THF is present as NaC5H5 · (THF)x, and it is generally necessary to know exactly how much of this NaC5H5 · (THF)x is present. This is accomplished by allowing a small amount of the NaC5H5 · (THF)x to react with water,
NaC5H5 · (C4H8O)x + H2O → NaOH(aq) + C5H5—H + x C4H8O
followed by titration of the NaOH(aq) with a standard acid. From the sample data tabulated below, determine the value of x in the formula NaC5H5 · (THF)x.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette





