The activity of a pure solid or liquid is approximately where V is the molar volume. For
Question:
The activity of a pure solid or liquid is approximately
![a = "V(P - P)` P] RT expl](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1700/4/8/4/480655b5580188e01.png)
where V̅ is the molar volume. For a pure solid or liquid under typical conditions, the quantity V̅(P - P°)/(RT) is quite small, primarily because V̅ is small, and so a ≈ 1. Use the following data, for liquid water at 300 K, to verify that a ≈ 1 over a wide range of pressures.
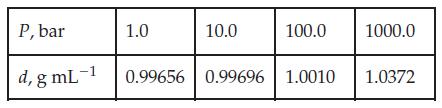
Transcribed Image Text:
a = "V(P - P°)` P⁹] RT expl
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)

Answered By

Mustafa olang
Please accept my enthusiastic application to solutionInn. I would love the opportunity to be a hardworking, passionate member of your tutoring program. As soon as I read the description of the program, I knew I was a well-qualified candidate for the position.
I have extensive tutoring experience in a variety of fields. I have tutored in English as well as Calculus. I have helped students learn to analyze literature, write essays, understand historical events, and graph parabolas. Your program requires that tutors be able to assist students in multiple subjects, and my experience would allow me to do just that.
You also state in your job posting that you require tutors that can work with students of all ages. As a summer camp counselor, I have experience working with preschool and kindergarten-age students. I have also tutored middle school students in reading, as well as college and high school students. Through these tutoring and counseling positions, I have learned how to best teach each age group. For example, I created songs to teach my three-year-old campers the camp rules, but I gave my college student daily quizzes to help her prepare for exams.
I am passionate about helping students improve in all academic subjects. I still remember my excitement when my calculus student received her first “A” on a quiz! I am confident that my passion and experience are the qualities you are looking for at solutionInn. Thank you so much for your time and consideration.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
1. How strong are the competitive forces confronting J. Crew in the market for specialty retail? Do a [Michael Porter] five-forces analysis to support your answer. (see chapter 3 in the textfor...
-
The term churn is very important to managers in the cellular phone business. Churning occurs when a customer stops using one companys service and switches to another companys service. Obviously,...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
1. Draw a flow chart for the following process You are approaching a local bank for getting an educational loan and draw a flow chart with all the process in detail and the decision node that you can...
-
Why is working capital management one of the most important and time-consuming activities of the financial manager? What is net working capital?
-
The Munchkin Theater is a nonprofit organization devoted to staging theater productions of plays for children in Toronto, Canada. The theater has a very small full-time professional administrative...
-
William Stem filed an action against Gary Braden, seeking to rescind a contract for the sale of an automobile and to obtain the return of the purchase price plus interest. The trial court granted...
-
Ken Lumas started his own consulting firm, Lumas Consulting, on June 1, 2014. The trial balance at June 30 is as follows. In addition to those accounts listed on the trial balance, the chart of...
-
A. Project of a building with 25 flats being evaluated for 15 years. Because of the growth for the city only 90% from the flats will be rented. This project has construction cost of 200000 and tand...
-
A classic experiment in equilibrium studies dating from 1862 involved the reaction in solution of ethanol (C 2 H 5 OH) and acetic acid (CH 3 COOH) to produce ethyl acetate and water. The reaction can...
-
For the reaction N 2 (g) + 3 H 2 (g) 2 NH 3 (g), the equilibrium constant is K p = 36.5 at 400 K. Two separate equilibrium mixtures have the following compositions at 400 K and a total pressure of...
-
Lancaster Wine Inc. receives an average of $19,000 in cheques per day. The delay in clearing is typically three days. The current interest rate is .017% per day. a. What is the companys collection...
-
How is differentiation parity different from cost parity? Multiple Choice Differentiation parity deals with pricing not innovation. Differentiation parity deals with innovation not value....
-
What are the fundamental differences between a monolithic kernel and a microkernel architecture, and how do these variances impact system performance and flexibility?
-
pwc.csod.com/lms/scorm/clientLMS/LMS3.aspx?rNum=1&aicc_sid=18846322... pwc.csod.com/lms/scorm/clientLMS/LMS3.aspx?rNum=1&aicc_sid=188463228p... pwc Prev O What is a key consideration to address...
-
Use the information contained below to compress one time unit per move using the Main Content ethod. Answer questions 64 through 67. Activity A B C D E F Predecessor None A A B D, E Duration 1 wk 3...
-
Below are the data for a Time-Cost CPM Scheduling model analysis. The time is in days and the costs include both direct and indirect costs. What are the total time of this project and total normal...
-
Explain the key issues in corporate governance as they relate to accounting
-
Why is a help desk and production support critical to system implementations? Discuss its interrelationship with the problem management and reporting system.
-
For each of the following payroll-related taxes, indicate whether they generally apply to (a) Employees only, (b) Employers only, or (c) Both employees and employers: 1. Federal income tax 2....
-
In a payroll system, what types of input data are referred to as (a) Constants (b) Variables?
-
To match revenues and expenses properly, should the expense for employee vacation pay be recorded in the period during which the vacation privilege is earned or during the period in which the...
-
Graphs, graph algorithms and methods, and graph theory are integral to IT and computer science applications and coding. For this assignment, What is a Hamiltonian cycle? What is a Euler cycle? What...
-
Elementary Graph Algorithms Illustrate how Search Algorithms work on a given graph G=(V,E) , starting at vertex A and using alphabetical vertex ordering . Search Algorithm B F F = Deepth-First...
-
In respect to limitations of the two dimensional nature of Modern Portfolio Theory, What time-series data can we effectively use in the model?

Study smarter with the SolutionInn App


