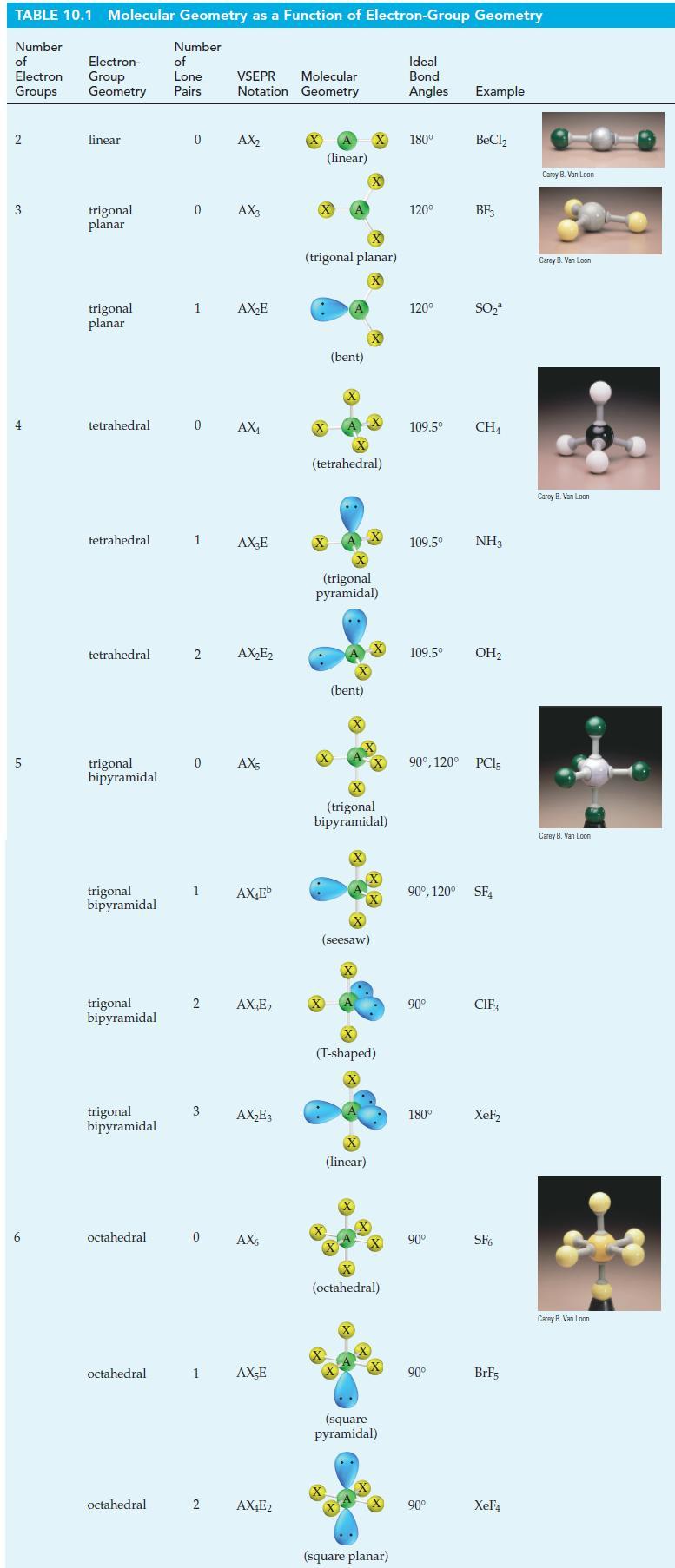
The molecular shape of BF 3 is planar (see Table 10.1). If a fluoride ion is attached
Question:
The molecular shape of BF3 is planar (see Table 10.1). If a fluoride ion is attached to the B atom of BF3 through a coordinate covalent bond, the ion BF4- results. What is the shape of this ion?
Table 10.1
Transcribed Image Text:
TABLE 10.1 Molecular Geometry as a Function of Electron-Group Geometry Number of Electron Groups 2 3 4 5 6 Electron- Group Geometry linear trigonal planar trigonal planar tetrahedral tetrahedral tetrahedral trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal trigonal bipyramidal octahedral octahedral octahedral Number of Lone Pairs 0 0 1 1 2 0 AX4 1 2 3 Molecular VSEPR Notation Geometry 0 AX₂ AX3 0 AX5 2 AX₂E AX₂E AX₂E₂ AX₂Eb AX₂E2 AX₂E3 AX6 1 AX-E AX4E2 (linear) X (trigonal planar) X X (bent) (tetrahedral) .. X (trigonal pyramidal) (bent) X X (seesaw) X X (trigonal bipyramidal) X (linear) (T-shaped) X X +4+ (octahedral) X X (square pyramidal) (square planar) Ideal Bond Angles Example 180⁰ 120⁰ 120⁰ 109.5⁰ 109.5⁰ 109.5⁰ NH3 90° 180° BeCl₂ 90° BF3 90⁰, 120° PC15 90° SO₂ 90⁰, 120° SF4 90° CH4 OH₂ CIF₁ XeF₂ SF6 BrF5 XeF4 Carey B. Van Loon Carey B. Van Loon Carey B. Van Loon Carey B. Van Loon Carey B. Van Loon
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
F F B BF FBF F F Answer Molecular and electron pair geometry for BF4 anion is Tetrahedral E...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The PMOS transistor in Fig. P5.41 has V=-0.5 V. As the gate voltage v, is varied from +3 V to 0 V, the transistor moves through all of its three possible modes of operation. Specify the values of v,...
-
For the system shown in Fig. first figure out what is the B matrix, then solve the linear power flow problem by setting up the relationship between P and 0 and using B' matrix. Find Bus 1 and Bus 2's...
-
If Sam gives a speech where he teaches his audience how to deal with a parking ticket, he is most likely using what organizational framework? Problem-Solution O Spatial Chronological O Cause-Effect O...
-
Taylor assaulted his wife, who then took refuge in Ms. Harringtons house. The next day, Mr. Taylor entered the house and began another assault on his wife, who knocked him down and, while he was...
-
A All-cis Cyclodecapentaene is a stable molecule that shows a single absorption in its 1H NMR spectrum at 5.67 ?. Tell whether it is aromatic, and explain its NMR spectrum.
-
Tom Bonacci brought his Jeep to Brewer Service Station to investigate a strange noise the vehicle was making. The Jeep was raised up on an automobile lift so that Brewer employee Paul Gebing could...
-
Presented below is an aging schedule for Sycamore Company. At December 31, 2014, the unadjusted balance in Allowance for Doubtful Accounts is a credit of $9,000. Instructions (a) Journalize and post...
-
Question 2 Consider a monopsonist with the following relationship between wage and labor: and the following production function w= a+bL f(K, L) = ln(L) + In(K) (3) (4) 1. Write the short run profit...
-
Use the VSEPR theory to predict the shape of (a) The molecule OSF 2 ; (b) The molecule O 2 SF 2 ; (c) The ion SF 5 - ; (d) The ion ClO 4 - ; (e) The ion ClO 3 - .
-
Use the VSEPR theory to predict the shapes of the anions (a) ClO 4 - ; (b) S 2 O 3 2- (that is, SSO 3 2- ); (c) PF 6 - ; (d) I 3 - .
-
Thermodynamically induced selective filtration describes the behavior of the hypothetical membrane for which K 0 = 1.0, solute activity coefficients are unity, and D sm / D wm = V w /V s . Partial...
-
US imposes a steep tariff on Chinese textiles raising the prices of t-shirts imported from China dramatically. What is the effect of this on the t-shirts made in the US?
-
1. "Develop a profile of a skilled or effective negotiator" 2. "Contrast a win-win negotiator with a win-lose negotiator" 3. What information should a buyer gather about a supplier before entering a...
-
Cultural competence is the ability to provide effective services cross-culturally. Diversity consciousness is a set of developed skills and awareness in the area of diversity. Diversity is present...
-
A suggested approach for effective presentation of a policy analysis report is to include highlights as: ( ) value judgement embodied in the cost-benefit technique ( ) value in the cost-benefit...
-
what is meant by self awareness, self concept and self esteem. What are the primary differences?
-
On January 1, 2013, the Haskins Company adopted the dollar-value LIFO method for its one inventory pool. The pools value on this date was $660,000. The 2013 and 2014 ending inventory valued at...
-
When a company has a contract involving multiple performance obligations, how must the company recognize revenue?
-
Responsibility and controllability. Consider each of the following independent situations: 1. A very successful salesman at Amcorp Computers regularly ignores the published sales catalog and offers...
-
Cash flow analysis, chapter appendix. (CMA, adapted) TabComp, Inc., is a retail distributor for MZB-33 computer hardware and related software and support services. TabComp prepares annual sales...
-
Budget schedules for a manufacturer. Sierra Furniture is an elite desk manufacturer. It makes two products: Executive desks'3' ?? 5' oak desks Chairman desks'6' ?? 4' red oak desks The budgeted...
-
Begin your initial post by introducing yourself to your classmates. Briefly share your thoughts on why you joined the class and what your short- and long-term goals are regarding financial...
-
1. Discuss the advantages and disadvantages of decentralization of a firm's operations. 2. What is the problem with using only financial measures of performance? 3. Why do managers analyze financial...
-
What is the primary focus of Capital Expenditure (CapEx) in cloud computing?

Study smarter with the SolutionInn App


