The N 2 O 4 NO 2 equilibrium mixture in the flask on the left in the
Question:
The N2O4–NO2 equilibrium mixture in the flask on the left in the figure is allowed to expand into the evacuated flask on the right. What is the composition of the gaseous mixture when equilibrium is re-established in the system consisting of the two flasks?
![]()
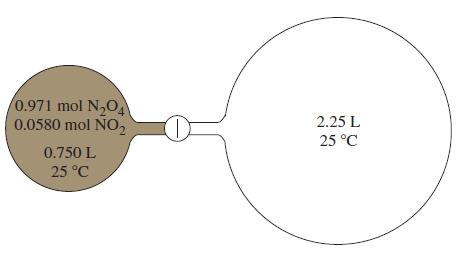
Transcribed Image Text:
N₂O4(g) 2 NO₂(g) K 4.61 x 10-3 at 25 °C =
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 40% (5 reviews)
NO48 Initially 2 NO2g moles Molarity M volume 0971 mol Molarity M 1...View the full answer

Answered By

Khurram shahzad
I am an experienced tutor and have more than 7 years’ experience in the field of tutoring. My areas of expertise are Technology, statistics tasks I also tutor in Social Sciences, Humanities, Marketing, Project Management, Geology, Earth Sciences, Life Sciences, Computer Sciences, Physics, Psychology, Law Engineering, Media Studies, IR and many others.
I have been writing blogs, Tech news article, and listicles for American and UK based websites.
4.90+
5+ Reviews
17+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
The following reaction is brought to equilibrium at 700 C. Indicate whether each of the following statements is true, false, or not possible to evaluate from the information given. (a) If the...
-
A 1.00 g sample of Ne(g) at 1 atm pressure and 27 C is allowed to expand into an evacuated vessel of 2.50 L volume. Does the gas do work? Explain.
-
Two options are available for reduction of pressure for superheated R-134a flowing steadily at 500 kPa and 40oC to a pressure of 100 kPa. In the first option, the vapor is allowed to expand through...
-
You have three light bulbs; bulb A has a resistance of 240 , bulb B has a resistance of 192 , and bulb C has a resistance of 144 . Each of these bulbs is used for the same amount of time in a setup...
-
Stewart Industries sells its finished product for $9 per unit. Its fixed operating costs are $20,000, and the variable operating cost per unit is $5. a. Calculate the firms earnings before interest...
-
Harris owned a farm that was worth about $600 per acre. By false representations of fact, Harris induced Pringle to buy the farm at $1,500 per acre. Shortly after taking possession of the farm,...
-
Steven Sanchez worked as a warehouse employee for Gruma Corporation. In December 2016, Sanchez was presented an arbitration agreement during a meeting with George Tate, the human resources manager...
-
Lovell Computer Parts Inc. is in the process of setting a selling price on a new component it has just designed and developed. The following cost estimates for this new component have been provided...
-
A wheel initially at rest begins rotating because of a constant angular acceleration. During a certain interval AT = 13s, the wheel goes through 84 rev and reaches an angular speed of 11 rev/s. (a)...
-
For the following reaction, K c = 2.00 at 1000 C. If a 5.00 L mixture contains 0.145 mol COF 2 , 0.262 mol CO 2 , and 0.074 mol CF 4 at a temperature of 1000 C, (a) Will the mixture be at...
-
Equilibrium is established in a 2.50 L flask at 250 C for the reaction How many moles of PCl 5 , PCl 3 , and Cl 2 are present at equilibrium, if (a) 0.550 mol each of PCl 5 and PCl 3 are initially...
-
Presented below are two independent situations. 1. On January 1, 2025, Simon Company issued $200,000 of 9%, 10-year bonds at par. Interest is payable quarterly on April 1, July 1, October 1, and...
-
You expect to receive $6,000 at graduation in two years. You plan on investing it at 11% until you have $67,000. How long will you wait from now? (Do not round intermediate calculations and round...
-
PowerShell Discuss how you can use PowerShell to manage Windows containers on a live network.
-
Compute the 2018 standard deduction for the following taxpayers. If an amount is zero, enter "0". Shonda is age 68 and single. She is claimed by her daughter as a dependent. Her earned income is...
-
27. Today is your 21st birthday and your parents gave you a gift of $2,000. You just put this money in a brokerage account, and your plan is to add $1,000 to the account each year on your birthday,...
-
Discuss the relationship between group policy design and security -What are some of the benefits and drawbacks associated with implementing group policies?
-
Global Graphics Company was organized on January 1, 2002. At the end of the first 6 months of operations, the trial balance contained the following accounts. Debit Balance Total .......$109,100...
-
Name some of the various types of financial intermediaries described in the chapter and indicate the primary reason(s) each was created.
-
Alpine Bicycle Company manufactures mountain bikes. The following data for May of the current year are available: Quantity of direct labor used 600 hrs. Actual rate for direct labor $12.50 per hr....
-
The Freedom Clothes Company produced 18,000 units during June of the current year. The Cutting Department used 3,500 direct labor hours at an actual rate of $12.10 per hour. The Sewing Department...
-
St. Luke Hospital began using standards to evaluate its Admissions Department. The standard was broken into two types of admissions as follows: The unscheduled admission took longer, since name,...
-
For df_calls, we will: Create new columns based on date, then join columns together to create columns such as SUB_YEAR_WEEK. We will create dummy variables for weekday names, months, and all call...
-
Do you agree with a Hacker's Code of Ethics? What is the difference between White Hat Hackers, Grey Hat Hackers, and Black Hat Hackers? Can someone justify breaking into a network without...
-
Writing a well-thought through, graduate-level paper of NOT LESS THAN four (4) pages, answer the following questions: 1.What is a context diagram? 2.When would you use a context diagram? 3.What are...

Study smarter with the SolutionInn App


