Question: The two compounds whose structures are depicted here are isomers. When derived from petroleum, they always occur mixed together. meta-Xylene is used in aviation fuels
The two compounds whose structures are depicted here are isomers. When derived from petroleum, they always occur mixed together. meta-Xylene is used in aviation fuels and in the manufacture of dyes and insecticides. The principal use of paraxylene is in the manufacture of polyester resins and fibers (for example, Dacron). Comment on the effectiveness of fractional distillation as a method of separating these two xylenes. What other method(s) might be used to separate them?
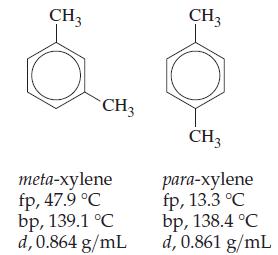
CH3 CH3 meta-xylene fp, 47.9 C bp, 139.1 C d, 0.864 g/mL CH3 CH3 para-xylene fp, 13.3 C bp, 138.4 C d, 0.861 g/mL
Step by Step Solution
3.35 Rating (161 Votes )
There are 3 Steps involved in it
Effectiveness of fractional distillation Fractional distillation is a method of separating liquids based on their different boiling points It is a ver... View full answer

Get step-by-step solutions from verified subject matter experts


