Use a cross-base arrow () to represent the polarity of the bond in each of the following
Question:
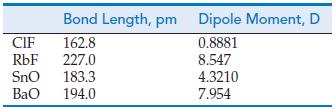
Use a cross-base arrow (⇸) to represent the polarity of the bond in each of the following diatomic molecules. Then use the data below to calculate, in the manner described, the partial charges (δ) on the atoms in each molecule. Express the partial charges as a decimal fraction of the elementary charge, e = 1.602 x 10-19 C, for example δ = +0.17e or δ = -0.17e.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





