Use the data here and in Table 7.2 to calculate f H of benzene, C 6
Question:
Use the data here and in Table 7.2 to calculate ΔfH° of benzene, C6H6(l).
![]()
Table 7.2
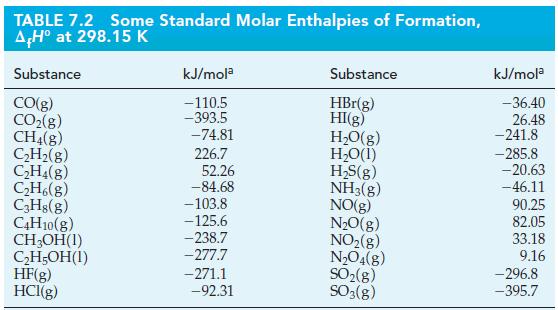
Transcribed Image Text:
2 C6H6(1) 15 O₂(g) 12 CO₂(g) + 6H₂O(1) A,H = -6535 kJ mol-1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Analyze We have a chemical equation and know the standard enthalpy of reaction We are asked ...View the full answer

Answered By

Usman Nasir
I did Master of Commerce in year 2009 and completed ACCA (Association of Chartered Certified Accountants) in year 2013. I have 10 years of practical experience inclusive of teaching and industry. Currently i am working in a multinational company as finance manager and serving as part time teacher in a university. I have been doing tutoring via many sites. I am very strong at solving numerical / theoretical scenario-based questions.
4.60+
16+ Reviews
28+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Use the data here to draw the supply and demand curves for a hamburger. You do not have to share your drawings; just reference them to answer the questions. Imagine a hamburger has the following...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Consider benzene (C6H6) in the gas phase. (a) Write the reaction for breaking all the bonds in C6H6 (g), and use data in Appendix C to determine the enthalpy change for this reaction. (b) Write a...
-
Which of the following is not a strategic disadvantage of vertical integration? Vertical integration poses all kinds of capacity-matching problems (achieving the most efficient scale of operation for...
-
Read the chapter opener about Hood River Juice Company. David Ryan explained that purchasing apples year-round and processing them immediately reduces costs, and that his company blends juices to fit...
-
Can we accurately say that, if something moves at constant velocity, there are no forces acting on it? Explain.
-
Hooters Restaurant in Myrtle Beach, South Carolina, used an alternative dispute resolution program, a program to resolve disputes outside the traditional court system. Employees of Hooters had to...
-
Raymond Companys trial balance at December 31, 2014, is presented below. All 2014 transactions have been recorded except for the items described shown below. Unrecorded transactions: 1. On May 1,...
-
The following are the ages of 13 mathematics teachers in a school district. 28, 30, 34, 34, 36, 38, 39, 42, 46, 47, 49, 50, 51 Notice that the ages are ordered from least to greatest. Give the...
-
On April 1, 2010, Jose Guadalupe established an interior decorating business, Lodge Designs. During the month, Jose completed the following transactions related to the business: Apr. 1. Jose...
-
The enthalpy of formation of formaldehyde is f H = -108.6 kJ/mol at 298.15 K. Write the chemical equation to which this value applies.
-
Let us apply equation (7.22) to calculate the standard enthalpy of combustion of ethane, C 2 H 6 (g), a component of natural gas. Eq. 7.22 A,H = [c x AHc + dx AHD +...] [ax AHA + bx AfHB +...] (7.22)...
-
a. An oil company acquires mining rights to a silver deposit. It is not obliged to mine the silver, however. The company has effectively acquired a __________ option, where the exercise price is the...
-
Dave lives with his wife, who is a full-time homemaker with no outside income. They have no dependents and file a joint return. Dave's mother, who is totally disabled and earns no income, lives with...
-
1. A 12-year loan of 14,000 is to be repaid with payments at the end of each year consisting of interest on the loan and a sinking fund deposit. Interest on the loan is charged at a 10.5% annual...
-
(Marginal normality and bivariate normality) Assume Y, Y2 ~iid N(0, 1). Consider the following two-dimensional vector: (1.2), if Y 0 (X1, X2) = { (Y,-2), if Y <0. Show that X1 and X2 are normally...
-
An accountant receives emails pertaining to three distinct categories: financial analysis requests, evaluation requests, and account payable requests. On any given day, 30% of the emails are...
-
A Canadian manufacturer imports 2,000 units of product worth USD$262.25 each from an American supplier. These imports are subject to GST on the equivalent Canadian value of the product. All of the...
-
The following payroll totals for the month of April are from the payroll register of Myth Corporation: salaries, $446,000; federal income taxes withheld, $62,880; Social Security tax withheld,...
-
How much more interest will be earned if $5000 is invested for 6 years at 7% compounded continuously, instead of at 7% compounded quarterly?
-
Entries for Held-to-Maturity Securities on January 1, 2009, Roosevelt Company purchased 12% bonds, having a maturity value of $500,000, for $537,907.40. The bonds provide the bondholders with a 10%...
-
Entries for Available-for-Sale Securities assume the same information as in E17-3 except that the securities are classified as available-for-sale. The fair value of the bonds at December 31 of each...
-
Effective-Interest versus Straight-Line Bond Amortization on January 1, 2010, Morgan Company acquires $300,000 of Nicklaus, Inc., 9% bonds at a price of $278,384. The interest is payable each...
-
4. For the graph shown below, describe and compare the instantaneous rate of change at the points indicated. Explain your reasoning.[6] Note: You do not necessarily need to calculate the...
-
Corporate law Topic :shares https://www.slideshare.net/syafawanimahadi/capital-shares-companies-act-2016 Rayyan Global Sdn Bhd's share capital comprises of RM40, 000 ordinary shares issued at RM5.00...
-
Based on these reviews of kakiyuki cafe, please apply servuction system (physical environment, the customer, and the service provider) to explain how well the service delivery is. 1. not enough...

Study smarter with the SolutionInn App


