Question: Use the information given here, data from Appendix D, and equation (7.22) to calculate the standard enthalpy of formation per mole of ZnS(s). Eq.7.22 2
Use the information given here, data from Appendix D, and equation (7.22) to calculate the standard enthalpy of formation per mole of ZnS(s).
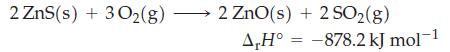
Eq.7.22
![A.H = [cx AHC + dx AHD +...] [ax AHA + bx AHB + .] (7.22) weighted sum of AcH values for the products](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/6/0/4/689654de8d1674691699604684867.jpg)
2 ZnS(s) + 3O(g) 2 ZnO(s) + 2 SO(g) A,H -878.2 kJ mol-1
Step by Step Solution
3.41 Rating (167 Votes )
There are 3 Steps involved in it
2ZnSs 302g 2ZnO2SO2g AH rxn... View full answer

Get step-by-step solutions from verified subject matter experts


