Use the Nernst equation and Table 19.1 to calculate E cell for each of the following cells.
Question:
Use the Nernst equation and Table 19.1 to calculate Ecell for each of the following cells.
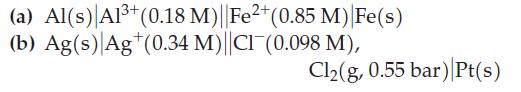
Table 19.1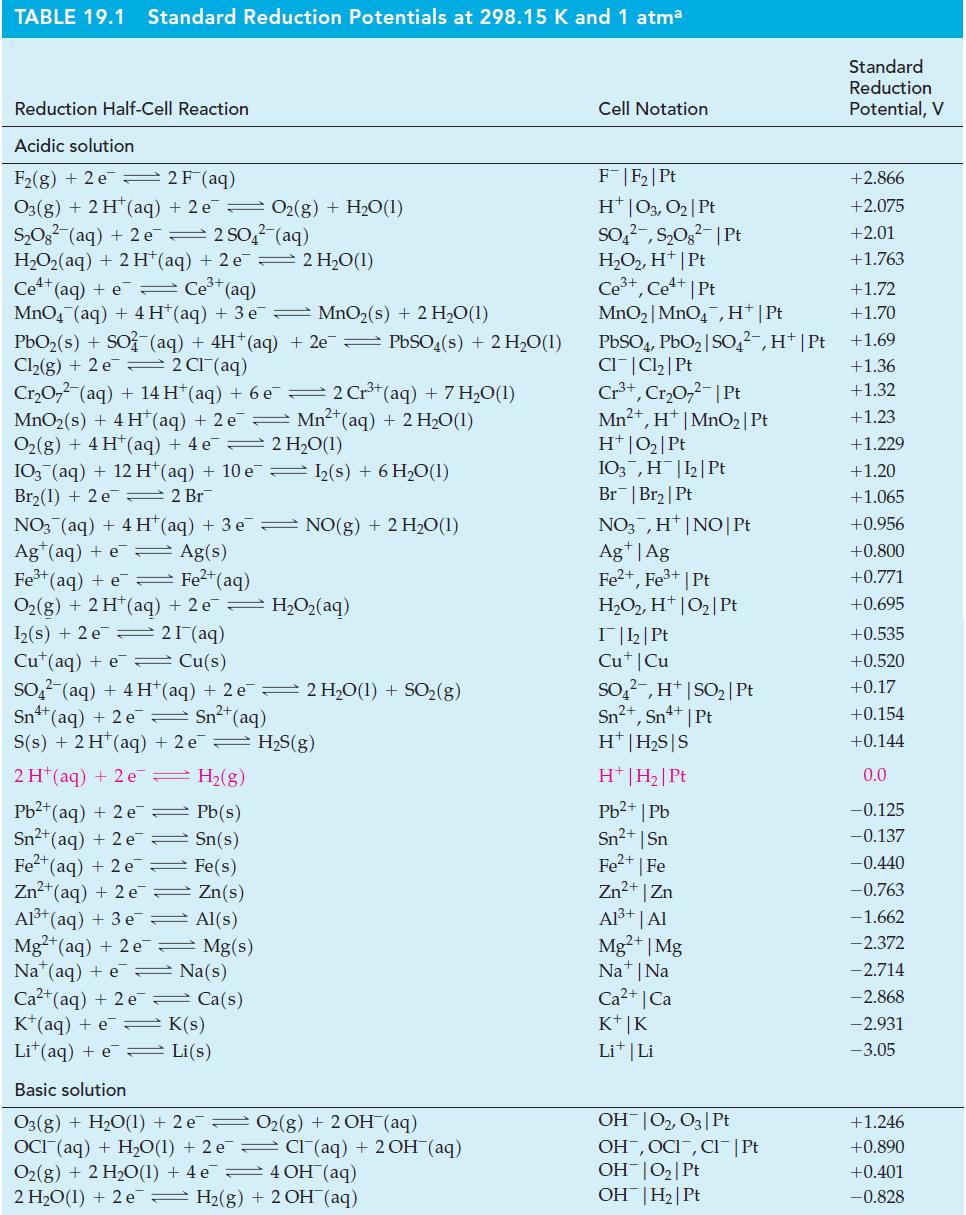
Transcribed Image Text:
(a) Al(s) Al³+ (0.18 M)||Fe2+ (0.85 M) Fe(s) (b) Ag(s) Ag (0.34 M)||CI (0.098 M), Cl₂(g, 0.55 bar) Pt(s)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 25% (4 reviews)

Answered By

Usman Nasir
I did Master of Commerce in year 2009 and completed ACCA (Association of Chartered Certified Accountants) in year 2013. I have 10 years of practical experience inclusive of teaching and industry. Currently i am working in a multinational company as finance manager and serving as part time teacher in a university. I have been doing tutoring via many sites. I am very strong at solving numerical / theoretical scenario-based questions.
4.60+
16+ Reviews
28+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
Determine the evaluative criteria that your target market(s) would use when choosing between alternative brands?
-
Use the Nernst equation and data from Appendix D to calculate E cell for each of the following cells. (a) Mn(s) Mn+ (0.40 M)||Cr+ (0.35 M), Cr+ (0.25 M) Pt(s) (b) Mg(s) Mg2+ (0.016 M)||[Al(OH)4](0.25...
-
Using the Nernst equation and the ion concentrations given in Table 121, calculate the equilibrium membrane potential of K + and Na + that is, the membrane potential where there would be no net...
-
CPA firm brings in a yoga instructor during the tax busy season to help relieve stress of the employees. Which is true about the CPA firm's ability to take a deduction for the yoga instructor's...
-
In many of the worlds marketplaces, a broad variety of media must be utilized to reach the majority of the market. Explain.
-
A backhoe acquired on January 5 at a cost of $84,000 has an estimated useful life of 12 years. Assuming that it will have no residual value, determine the depreciation for each of the first two years...
-
Does Fairmont have any contactor personnel whose have terminated but are being paid through payroll after termination (e.g., ghost employees)?
-
Fair Value and Equity Method Compared) Gregory Inc. acquired 20% of the outstanding common stock of Henderson Inc. on December 31, 2010. The purchase price was $1,250,000 for 50,000 shares. Henderson...
-
You will be taking over the vending machine business at UNCC from Tony. You are selling 20-ounce bottles of Dasani bottled water for $1.25, and Tony will give you a large stock of Dasani to get you...
-
A voltaic cell represented by the following cell diagram has E cell = 1.250 V. What must be [Ag + ] in the cell? 2+ Zn(s) Zn+ (1.00 M)||Ag+ (x M) Ag(s)
-
Consider the reduction half-cell reactions listed in Appendix D, and give plausible explanations for the following observations: (a) For some half-cell reactions E depends on pH; for others, it does...
-
What is ecological succession? How does primary and secondary succession differ?
-
make your final deicison which is will you not buy a car so you can use public transportation and save money, ect. or will you buy the 2015 BMW i3 electric car (total montly cost $635.42) so you can...
-
Wealth ended malaria in the United States by: enabling people to move out of the country. enabling the society to pay for preventive measures. slowing down economic growth. enabling the United States...
-
I've considered how inflation affects future value investments. Inflation causes less spending and lowers investor's interest and is one of the top 2 disadvantages however currency exposure can...
-
1. One common Physical characteristic of toddler development that can foster frustration for family/child.
-
Calculate the Mean Length of Utterance (MLU) for this sample . Please show your work, counting the morphemes utterance by utterance. Do this by typing out one utterance per line, and typing out the...
-
Critics of SFAS No. 87 argue that its requirements result in reporting pension expense that is volatile. One of the factors causing volatility is changing the discount rate used to calculate service...
-
What are the principal differences among asset liquidity management, liability management, and balanced liquidity management?
-
Indicate whether each of the following would be added to or deducted from net income in determining net cash flow from operating activities by the indirect method: (a) Decrease in accounts receivable...
-
The net income reported on the income statement for the current year was $132,000. Depreciation recorded on store equipment for the year amounted to $21,800. Balances of the current asset and current...
-
The income statement disclosed the following items for 2010: Depreciation expense $ 36,000 Gain on disposal of equipment 21,000 Net income 317,500 Balances of the current assets and current liability...
-
A sports car accelerates from 13.85 meters per second to 31.52 meters per second in 6.61 seconds. How far does the car travel (in meters) in this time?
-
8. A single-phase AC supply has ac mains voltage of 220 V at 50Hz and feeder (source) impedance of 3.0 ohms inductive reactance (Zs=j3) after which a single-phase load Z= 13.2 +19) ohms is connected...
-
The table below provides the price and dividend information of BMI stock in 2020. What is the cumulative return of the BMI stock in 2020? Date Stock Price Dividend 1/1/2020 $36 3/25/2020 $40 $0.60...

Study smarter with the SolutionInn App


