Using methods similar to Examples 12-10 and 12-11, calculate the density of CsCl. Use 169 pm as
Question:
Using methods similar to Examples 12-10 and 12-11, calculate the density of CsCl. Use 169 pm as the radius of Cs+.
Examples 12-10
Use data from Example 12-9, together with the molar mass of Fe and the Avogadro constant, to calculate the density of iron.
Example 12-9
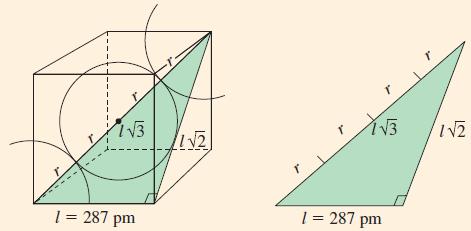
At room temperature, iron crystallizes in a bcc structure. By X-ray diffraction, the edge of the cubic cell corresponding to Figure 12-45 is found to be 287 pm. What is the radius of an iron atom?
Figure 12-45
Examples 12-11
The ionic radii of Na+ and Cl- in NaCl are 99 and 181 pm, respectively. What is the edge length of the unit cell?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





