Question: Use data from Example 12-9, together with the molar mass of Fe and the Avogadro constant, to calculate the density of iron. Example 12-9 At
Use data from Example 12-9, together with the molar mass of Fe and the Avogadro constant, to calculate the density of iron.
Example 12-9
At room temperature, iron crystallizes in a bcc structure. By X-ray diffraction, the edge of the cubic cell corresponding to Figure 12-45 is found to be 287 pm. What is the radius of an iron atom?
Figure 12-45
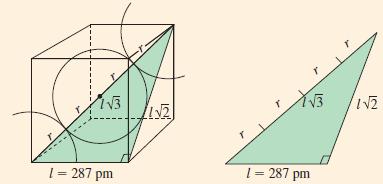
13 1 = 287 pm W21 YB 1 = 287 pm IN2
Step by Step Solution
3.46 Rating (156 Votes )
There are 3 Steps involved in it
Analyze To calculate the density we need the mass of the unit cell in grams and its volume in cm3 Fr... View full answer

Get step-by-step solutions from verified subject matter experts


