(A) Use the result of Practice Example 12-9A, the molar mass of K, and the Avogadro constant...
Question:
(A) Use the result of Practice Example 12-9A, the molar mass of K, and the Avogadro constant to calculate the density of potassium.
(B) Use the result of Practice Example 12-9B, the molar mass of Al, and its density (2.6984 g cm-3) to evaluate the Avogadro constant, NA.
Example 12-9A
(A) Potassium crystallizes in the bcc structure. What is the length of the unit cell in this structure? Use the metallic radius of potassium given in Figure 9-11.
Figure 9-11
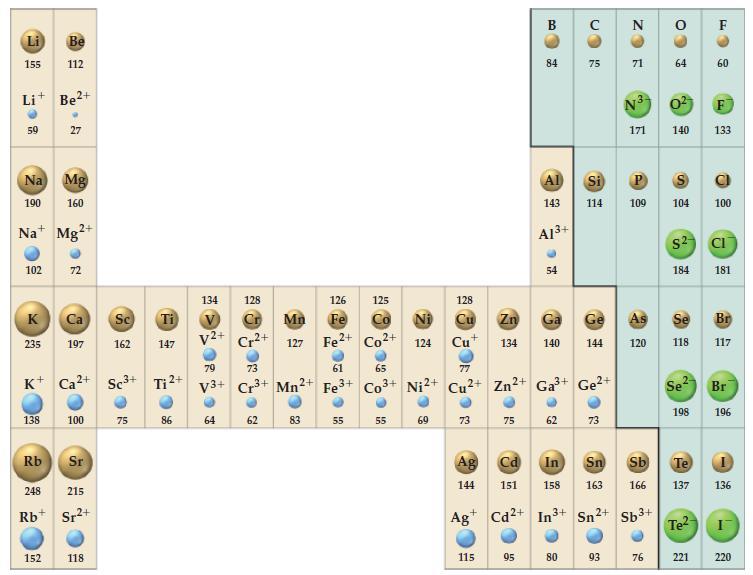
Example 12-9B
(B) Aluminum crystallizes in an fcc structure. Given that the atomic radius of Al is 143.1 pm, what is the volume of a unit cell?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





