Werner demonstrated that octahedral complexes can exhibit optical isomerism, and to Werners satisfaction, this confirmed the octahedral
Question:
Werner demonstrated that octahedral complexes can exhibit optical isomerism, and to Werner’s satisfaction, this confirmed the octahedral arrangement of ligands. However, skeptics of his theory said that because the ligands contained carbon atoms, he could not rule out carbon as the source of the optical activity. Werner devised and prepared the following compound in which the OH– groups act as bridging groups.
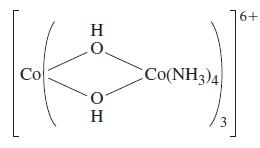
Werner resolved this compound into its optical isomers, confirming his theory and confounding his critics. What are the oxidation states of the Co ions? If the complex is low spin, what is the number of unpaired electrons in the molecule? Draw the structures of the two optical isomers.
Step by Step Answer:

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette




