What concentration of formate ion, [HCOO - ] should be present in 0.366 M HCOOH to produce
Question:
What concentration of formate ion, [HCOO-] should be present in 0.366 M HCOOH to produce a buffer solution with pH = 4.06?
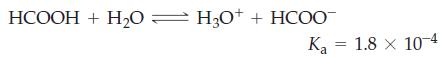
Transcribed Image Text:
HCOOH + H2O=H3O* + HCOO- Ka 1.8 x 10-4
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To solve this problem we can use the HendersonHasselbalch equation for ...View the full answer

Answered By

Vineet Kumar Yadav
I am a biotech engineer and cleared jee exam 2 times and also i am a math tutor. topper comunity , chegg India, vedantu doubt expert( solving doubt for iit jee student on the online doubt solving app in live chat with student)
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:
Students also viewed these Sciences questions
-
What concentration of ammonia, [NH 3 ], should be present in a solution with [NH 4 + ] = 0.732 M to produce a buffer solution with pH = 9.12? For NH 3 , K b = 1.8 x 10 -5 .
-
You work in a research lab with a chemist who asks you to make 500.0 mL of a formic acid/sodium formate buffer solution with pH = 4.10. Formic acid is HCOOH(aq); sodium formate is NaHCOO(s) and is...
-
What mass of sodium formate must be added to 500.0 mL of 1.00 M formic acid to produce a buffer solution that has a pH of 3.50?
-
An investor bought a 70-strike European put option on an index with six months to expiration.The premium for this option was 1. The investor also wrote an 80-strike European put optionon the same...
-
In what financial activities does a corporate treasurer engage?
-
Historically, Pine Hill Wood Products has had no significant bad debt experience with its customers. There are no cash sales; all sales are made on credit. Payments for credit sales have been...
-
In 2016, a hacker tricked a Lamps Plus employee into disclosing the tax information of approximately 1,300 other employees, resulting in the filing of a fraudulent income tax return of Lamps Plus...
-
Everett Co. was organized on July 1, 2015. Quarterly financial statements are prepared. The unadjusted and adjusted trial balances as of September 30 are shown below. Instructions (a) Journalize the...
-
Pops Popcorn has three project choices for the coming year, but only $9,000 in its budget for new projects. Project 1 is a new corn seed separator that identifies grannies (seeds that do not pop when...
-
(A) A 1.00 L volume of buffer is made with concentrations of 0.350 M NaHCOO (sodium formate) and 0.550 M HCOOH (formic acid). (a) What is the initial pH? (b) What is the pH after the addition of...
-
Calculate [OH - ] in a solution that is (a) 0.0062 M Ba(OH) 2 and 0.0105 M BaCl 2 ; (b) 0.315 M (NH 4 ) 2 SO 4 and 0.486 M NH 3 ; (c) 0.196 M NaOH and 0.264 M NH 4 Cl.
-
Milena owns a 25% interest in Davis Company, an S corporation. Her basis in the Davis stock is $40,000. Davis reports an operating loss of $200,000 in the current year. Davis owes Milena $25,000 on a...
-
In the 21st century, the study of Ecclesiology has now taken center stage. In general, the success and proliferation of alternative church arrangements through the use of technology, namely...
-
The following statements have errors. What are they? class Person { }; private: string name; string address; A constructor with two parameters public: void Person (string n, string a) { } name = n;...
-
Working in teams of female and male members, develop a list of behaviors that could be classified as quid pro quo harassment or hostile environment. Explore the possibility that some sexual harassing...
-
A new engineer is evaluating whether to use a larger-diameter pipe for a water line. It will cost $350,000 more initially, but it will reduce pump- ing costs. The optimistic, most likely, and pes-...
-
1. What is a make file and what is it used for in software development? 2. Describe in your own words what each line of the below make file does. tempconv: tempconv.o g++ -o tempconv tempconv.o...
-
There are 12 members on the board of directors for Cliffside General Hospital. a. If they must elect a chairperson, first vice chairperson, second vice chairperson, and secretary, how many different...
-
A Alkynes can be made by dehydrohalogenation of vinylic halides in a reaction that is essentially an E2 process. In studying the stereochemistry of this elimination, it was found that...
-
The Home Depot reported the following data (in millions) in its financial statements: a. Determine the ratio of net sales to average total assets for The Home Depot for 2007 and 2006. Round to two...
-
Kroger, a national supermarket chain, reported the following data (in millions) in its financial statements for 2007: Total revenue $66,111 Total assets at end of year 21,215 Total assets at...
-
The following selected accounts and their current balances appear in the ledger of Case-It Co. for the fiscal year ended November 30, 2010: 1. Prepare a multiple-step income statement.2. Prepare a...
-
Profit Kit I Kit II Kit III 1 320 2205 2310 10 15 18 A pet store sells three different starter kits for 10-gallon aquariums. The accompanying chart shows the contents of each kit. The store has 84...
-
Research and review the following article: Harel, A . , & Harpaz, G . ( 2 0 2 1 ) . Forecasting stock prices. International Review of Economics & Finance, 7 3 , 2 4 9 2 5 6 . provide a detailed...
-
Multiply. 7 12 20 49 7 12 20 49 = (Type an integer or a simplified fraction.)

Study smarter with the SolutionInn App


