When the ionization energies of a series of isoelectronic atoms and ions are compared, an interesting relationship
Question:
When the ionization energies of a series of isoelectronic atoms and ions are compared, an interesting relationship is observed for some of them. In particular, if the square root of the ionization energy (in kJ mol-1) for the series Li, Be+, B2+, C3+, N4+, O5+, and F6+ is plotted against the atomic number (Z) of the species, a linear relationship is obtained. The corresponding graph for the series Na, Mg+, Al2+, Si3+, P4+, S5+, and Cl6+ is also linear. The graph is shown below.
The equations for the two lines joining the points are
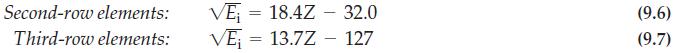
Explain the origin of these relationships and the differences in the numerical coefficients.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

General Chemistry Principles And Modern Applications
ISBN: 9780132931281
11th Edition
Authors: Ralph Petrucci, Jeffry Madura, F. Herring, Carey Bissonnette
Question Posted:





