A mixture of ethanol and water vapor is being rectified in an adiabatic distillation column. The alcohol
Question:
A mixture of ethanol and water vapor is being rectified in an adiabatic distillation column. The alcohol is vaporized and transferred from the liquid to the vapor phase. Water vapor condenses-enough to supply the latent heat of vaporization needed by the alcohol being evaporated-and is transferred from the vapor to the liquid phase. Both components diffuse through a gas film \(0.1 \mathrm{~mm}\) thick. The temperature is \(368 \mathrm{~K}\) and the pressure is \(1 \mathrm{~atm}\). The mol fraction of ethanol is 0.8 on one side of the film and 0.2 on the other side of the film. Calculate the rate of diffusion of ethanol and of water, in \(\mathrm{kg} / \mathrm{m}^{2} \cdot \mathrm{s}\). The latent heat of vaporization of the alcohol and water at \(368 \mathrm{~K}\) can be estimated by the Pitzer acentric factor correlation (Reid et al., 1987)
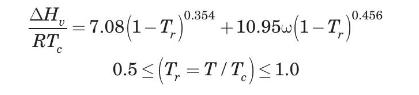
where \(\omega\) is the acentric factor.
Step by Step Answer:






