Benzene vapor in the gaseous effluent of an industrial process is scrubbed with a wash oil in
Question:
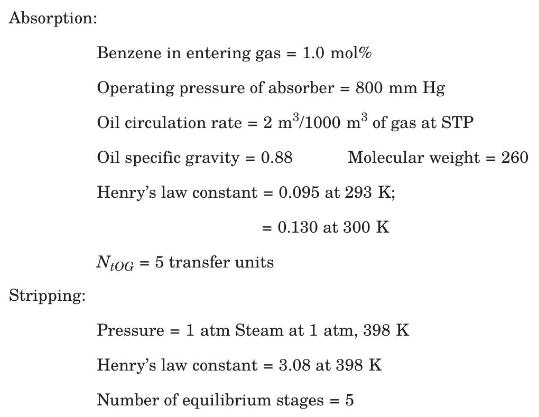
Benzene vapor in the gaseous effluent of an industrial process is scrubbed with a wash oil in a countercurrent packed absorber. The resulting benzene-wash oil solution is then heated to \(398 \mathrm{~K}\) and stripped in a tray tower, using steam as the stripping medium. The stripped wash oil is then cooled and recycled to the absorber. Some data relevant to the operation follow:
(a) In the winter, it is possible to cool the recycled oil to \(293 \mathrm{~K}\), at which temperature the absorber then operates. Under these conditions \(72.0 \mathrm{~kg}\) of steam is used in the stripper per \(1000 \mathrm{~m}^{3}\) of gas at STP entering the absorber. Calculate the percent benzene recovery in the winter.
(b) In the summer, it is impossible to cool the recycled wash oil to lower than \(300 \mathrm{~K}\) with the available cooling water. Assuming that the absorber then operates at \(300 \mathrm{~K}\), with the same oil and steam rates, and that \(N_{t O G}\) and equilibrium stages remain the same, what summer recovery of benzene can be expected?
(c) If the oil rate cannot be increased, but the steam rate in the summer is increased by \(50 \%\) over the winter value, what summer recovery of benzene can be expected?
Step by Step Answer:






