A tarnished silver knife is placed in a beaker containing hot aqueous NaHCO 3 . A piece
Question:
A tarnished silver knife is placed in a beaker containing hot aqueous NaHCO3. A piece of Al foil is placed in the solution so that it touches the knife. The deposit of Ag2S disappears and the clean knife is recovered from the solution.
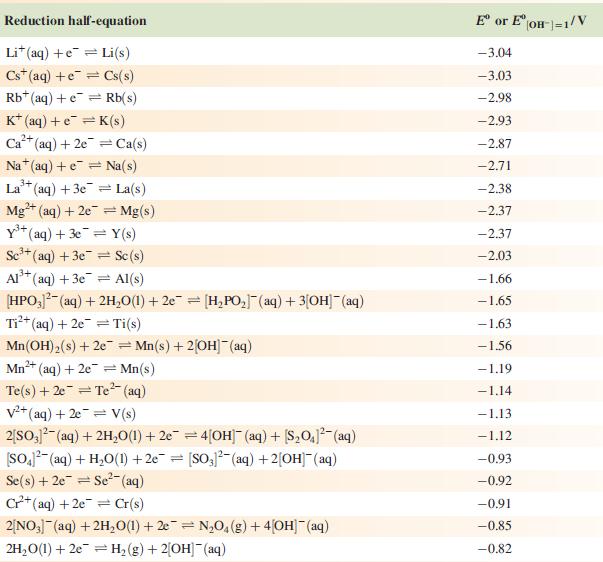
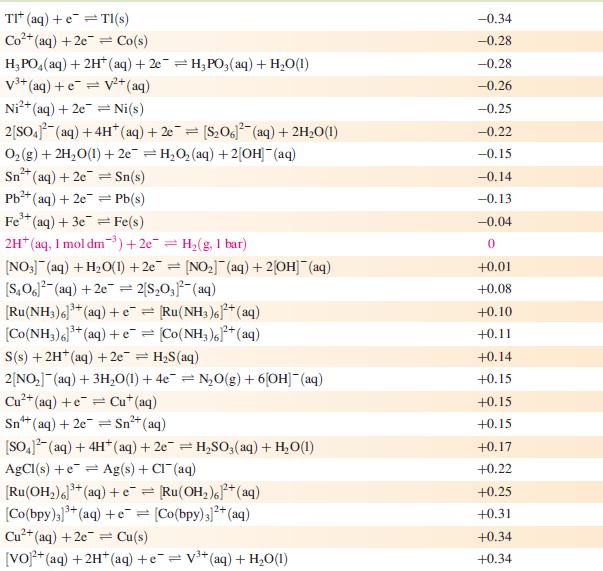
(a) Using Appendix 11 and the data given below, write an equation for the electrochemical process that cleans the knife, and determine E°cell and ΔG°cell (298 K).
(b) Al2S3 is decomposed by water. Write an equation to show what happens.
(c) Write an equation for the equilibria set up when NaHCO3 is added to H2O. Suggest two reasons why aqueous NaHCO3 rather than distilled water is used in the cleaning process.
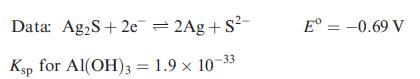
Data from Appendix 11
The concentration of each aqueous solution is 1 mol dm−3 and the pressure of a gaseous component is 1 bar (105 Pa). (Changing the standard pressure to 1 atm (101 300 Pa) makes no difference to the values of E° at this level of accuracy.) Each half-cell listed contains the specified solution species at a concentration of 1 mol dm−3; where the half-cell contains [OH]−, the value of E° refers to [OH−] = 1 mol dm−3, hence the notation E°[OH−] = 1

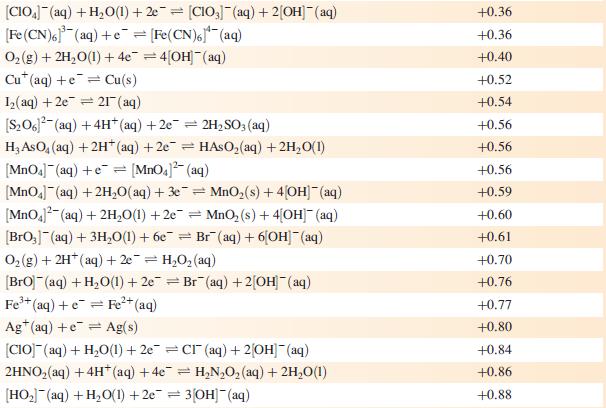

Step by Step Answer:






