MLCT bands can be recognized by the fact that the energy is a sensitive function of the
Question:
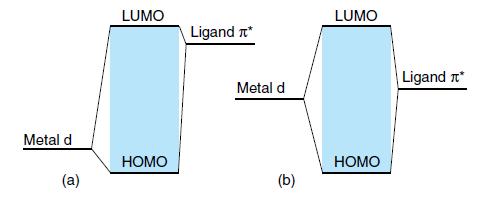
MLCT bands can be recognized by the fact that the energy is a sensitive function of the polarity of the solvent (because the excited state is more polar than the ground state). Two simplified molecular orbital diagrams are shown in Fig. 20.45.
(a) Is a case with a ligand π level higher than the metal d orbital.
(b) Is a case in which the metal d orbital and the ligand level are at the same energy.
Which of the two MLCT bands should be more solvent-sensitive? These two cases are realized by [W(CO)4(phen)] and [W(CO)4(iPr-DAB)], respectively, where DAB = 1,4-diaza- 1,3-butadiene.
Comment on the CT character of the transition as a function of the extent of backdonation by the metal atom.
Figure 20.45.
Step by Step Answer:

Inorganic Chemistry
ISBN: 9780198768128
7th Edition
Authors: Mark Weller, Tina Overton, Jonathan Rourke





