The first step in the Cativa process is the reaction between MeI and cis-[Ir(CO) 2 I 2
Question:
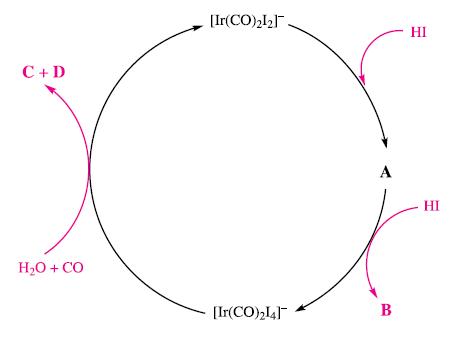
The first step in the Cativa process is the reaction between MeI and cis-[Ir(CO)2I2]−. However, the catalyst may also react with HI and this step initiates a water gas shift reaction that competes with the main catalytic cycle.
(a) What chemical is manufactured in the Cativa process? Why is this product of industrial importance?
(b) Why is HI present in the system?
(c) Give an equation for the water gas shift reaction, and state conditions typically used in industry.
(d) Figure 25.21 shows the competitive catalytic cycle described above.
Suggest identities for species A, B, C and D. What type of reaction is the conversion of cis-[Ir(CO)2I2]− to A? What changes in iridium oxidation state occur on going around the catalytic cycle, and what is the electron count in each iridium complex?
Figure 25.21
Step by Step Answer:






