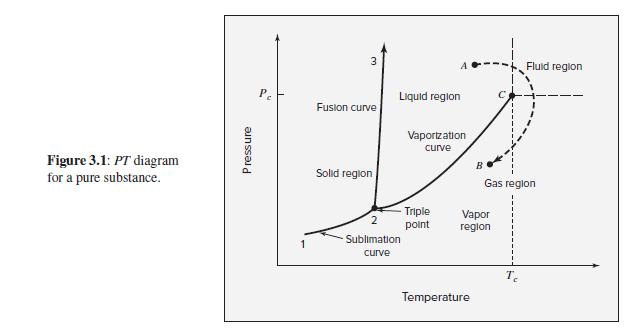
As suggested by Fig. 3.1, the slope of the sublimation curve at the triple point is generally
Question:
As suggested by Fig. 3.1, the slope of the sublimation curve at the triple point is generally greater than that of the vaporization curve at the same state. Rationalize this observation. Note that triple-point pressures are usually low; hence assume for this exercise that Δ Zsv ≈ Δ Zlv ≈ 1.
Fig. 3.1
Transcribed Image Text:
Fluid reglon Liquid region Fusion curve Vaportzation curve Figure 3.1: PT diagram for a pure substance. Solid region Gas region Triple Vapor region point Sublimation curve Temperature Pressure
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 57% (7 reviews)
The slope of the sublimation curve at the triple point is generally greater than that of the vaporiz...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart
Question Posted:
Students also viewed these Engineering questions
-
Point a in Fig is maintained at a constant potential of 400 V. above ground. (See Problem) (a) What is the reading of a voltmeter with the proper range and with resistance 5.00 X 104 Ω when...
-
Assume the same data as in Appendix 1 Exercise 12-19, Except that the current interest rate is 12%. In Appendix 1 Exercise 12-19, On January 1, 2014, you win $75,000,000 in the state lottery. The...
-
At what point on the curve y = 1 + 2ex 3x is the tangent line parallel to the line 3x y = 5? Illustrate by graphing the curve and both lines.
-
The objective of this problem is to design and develop a program for Huffman coding algorithm. The discrete source has an alphabet X = {x1, x2, x3, x4, x5, x6, x7, x8, x9} with corresponding...
-
List the information required for accurate meeting minutes.
-
Dougs Diner is planning to expand operations and is concerned that its reporting system might need improvement. The master budget income statement for the Downtown Dougs, which contains a...
-
Using the software development life cycle approach, do we apply project management principles to software development? Elaborate on your answer.
-
What is the value of a preferred stock where the dividend rate is 14 percent on a $100 par value, and the markets required yield on similar shares is 12 percent?
-
What was an effective part of the content of the speech? What was the most effective part of the delivery of the speech? How could content be improved? How could delivery be improved?
-
In 2005, Bob Moyer was reviewing production costs for Mile High Cycles. Located in Denver, Colorado, the company sold very high-quality, handcrafted mountain bikes to bicycle retailers throughout the...
-
An ideal gas with constant heat capacities enters a converging/diverging nozzle with negligible velocity. If it expands isentropically within the nozzle, show that the throat velocity is given by:...
-
Real-gas behavior for turbomachinery is sometimes empirically accommodated through the expression W = Z W ig , where W ig is the ideal-gas mechanical power and Z is some suitably defined average...
-
On January 12, 2016, Washington Company purchased a computer (cost, $13,000; expected life five years; estimated salvage value , $1,500) and a lightweight van for delivery purposes (cost, $65,500;...
-
Write a program FourSum that reads Tong integers from standard input, and counts the number of 4-tuples that sum to zero. Use a quadruple nested loop. What is the order of growth of the running time...
-
Modify Index to make a program IndexLines that considers only consecutive sequences of letters as keys (no punctuation or numbers) and uses line number instead of word position as the value. This...
-
Show by approximating with integrals that the number of distinct triples of integers between 0 and \(n\) is about \(n^{3} / 6\).
-
Describe why it is desirable to use immutable keys with binary search.
-
Add to Graph a method hasVertex() that takes a string argument and returns true if it names a vertex in the graph, and false otherwise.
-
Show that the set of complex numbers x + i y forms a real vector space under the operations of addition (x + iy) + (w + i v) = (x + u) + i (y + v) and scalar multiplication c(x + iy) = cx + i cy....
-
14. In testing the existence assertion, an auditor ordinarily works from the a. Financial statements to the accounting records. b. General journal to the general ledger. c. Supporting evidence to the...
-
The Reynolds number Re is a dimensionless group that characterizes the intensity of a flow. For large Re, a flow is turbulent; for small Re, it is laminar. For pipe flow, Re uD/, where D is pipe...
-
A list of common unit operations follows: (a) Single-pipe heat exchanger (b) Double-pipe heat exchanger (c) Pump (d) Gas compressor (e) Gas turbine (f) Throttle valve (g) Nozzle Develop a simplified...
-
A solid body at initial temperature T 0 is immersed in a bath of water at initial temperature T w0 . Heat is transferred from the solid to the water at a rate Q = K (T w T) , where K is a constant...
-
If you weigh your portfolio in 80% risky and 20% risk-free t bills. The optimal risky portfolio consists of 40% in bonds and 60% in ETFs. How much will you have invested in t-bills, bonds, and etfs?
-
b. A 25-year mortgage loan of $184,000 at a 10 percent compound annual interest rate with equal installment payments at the end of each year.
-
What are Cloud-based Services? How does this differ to the common virtualization model?

Study smarter with the SolutionInn App


