Generate P-x 1 -y 1 diagrams at 100C for one of the systems identified below. Base activity
Question:
Generate P-x1-y1 diagrams at 100°C for one of the systems identified below. Base activity coefficients on the Wilson equation, Eqs. (13.45) to (13.47). Use two procedures: (i) modified Raoult’s law, Eq. (13.19), and (ii) the gamma/phi approach, Eq. (13.13), with Φi given by Eq. (13.14). Plot the results for both procedures on the same graph. Compare and discuss them.
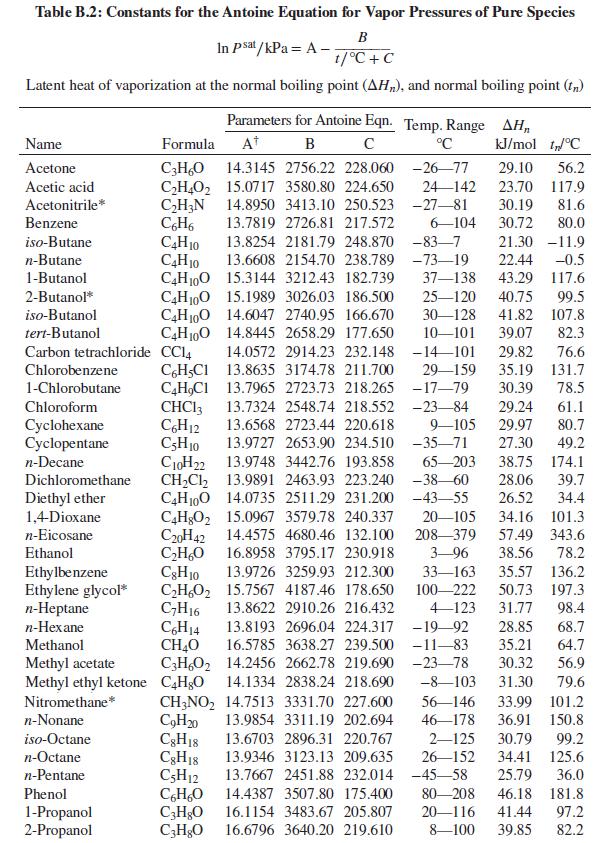
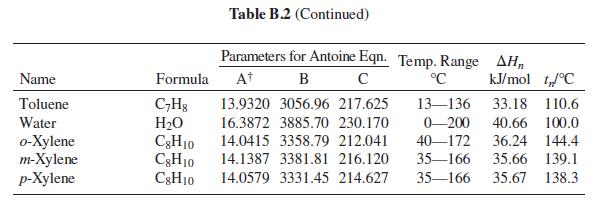
Data sources: For Psati use Table B.2. For vapor-phase nonidealities, use material from Chap. 3; assume that the vapor phase is an (approximately) ideal solution. Estimated parameters for the Wilson equation are given for each system.
(a) Benzene(1)/carbon tetrachloride(2): Λ12 = 1.0372, Λ21 = 0.8637
(b) Benzene(1)/cyclohexane(2): Λ12 = 1.0773, Λ21 = 0.7100
(c) Benzene(1)/n-heptane(2): Λ12 = 1.2908, Λ21 = 0.5011
(d) Benzene(1)/n-hexane(2): Λ12 = 1.3684, Λ21 = 0.4530
(e) Carbon tetrachloride(1)/cyclohexane(2): Λ12 = 1.1619, Λ21 = 0.7757
(f) Carbon tetrachloride(1)/n-heptane(2): Λ12 = 1.5410, Λ21 = 0.5197
(g) Carbon tetrachloride(1)/n-hexane(2): Λ12 = 1.2839, Λ21 = 0.6011
(h) Cyclohexane(1)/n-heptane(2): Λ12 = 1.2996, Λ21 = 0.7046
(i) Cyclohexane(1)/n-hexane(2): Λ12 = 1.4187, Λ21 = 0.5901
Eq (13.45)
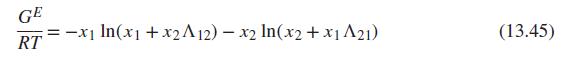
Eq (13.47)
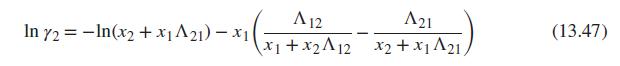
(i) Eq (13.19)

(ii) Eq (13.13)
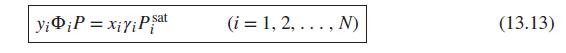
(ii) Eq (13.14)

Table B.2
Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





