The relative volatility 12 is commonly used in applications involving binary VLE. In particular (see Ex.
Question:
The relative volatility α12 is commonly used in applications involving binary VLE. In particular (see Ex. 13.1), it serves as a basis for assessing the possibility of binary azeotropy. (a) Develop an expression for α12 based on Eqs. (13.13) and (13.14). (b) Specialize the expression to the composition limits x1 = y1 = 0 and x1 = y1 = 1. Compare with the result obtained from modified Raoult’s law, Eq. (13.19). The difference between the results reflects the effects of vapor-phase nonidealities. (c) Further specialize the results of part (b) to the case where the vapor phase is an ideal solution of real gases.
(Ex. 13.1)
For the system methanol(1)/methyl acetate(2), the following equations provide a reasonable correlation for the activity coefficients:
![]()
In addition, the following Antoine equations provide vapor pressures:
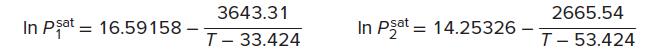
where T is in kelvins and the vapor pressures are in kPa. Assuming the validity of Eq. (13.19), calculate:
(a) P and { yi} for T = 318.15 K and x1 = 0.25.
(b) P and {xi} for T = 318.15 K and y1 = 0.60.
(c) T and { yi} for P = 101.33 kPa and x1 = 0.85.
(d) T and {xi} for P = 101.33 kPa and y1 = 0.40.
(e) The azeotropic pressure and the azeotropic composition for T = 318.15 K.
Eq. (13.19)

(a) Eq (13.13)
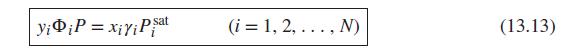
(a) Eq (13.14)

Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





