Reaction-equilibrium calculations may be useful for estimation of the compositions of hydrocarbon feedstocks. A particular feedstock, available
Question:
Reaction-equilibrium calculations may be useful for estimation of the compositions of hydrocarbon feedstocks. A particular feedstock, available as a low-pressure gas at 500 K, is identified as “aromatic C8.” It could in principle contain the C8H10 isomers: o-xylene (OX), m-xylene (MX), p-xylene (PX), and ethylbenzene (EB). Estimate how much of each species is present, assuming the gas mixture has come to equilibrium at 500 K and low pressure. The following is a set of independent reactions (why?):
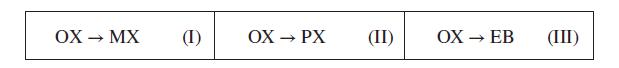
(a) Write reaction-equilibrium equations for each equation of the set. State clearly any assumptions.
(b) Solve the set of equations to obtain algebraic expressions for the equilibrium vapor-phase mole fractions of the four species in relation to the equilibrium constants, KI, KII, KIII.
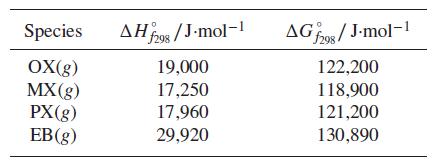
(c) Use the following data to determine numerical values for the equilibrium constants at 500 K. State clearly any assumptions.
(d) Determine numerical values for the mole fractions of the four species.
Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





