The first step in the metabolism of ethanol is dehydrogenation by reaction with nicotinamide-adenine dinucleotide (NAD): C
Question:
The first step in the metabolism of ethanol is dehydrogenation by reaction with nicotinamide-adenine dinucleotide (NAD):
C2H5OH + NAD+ → C2H4O + NADH
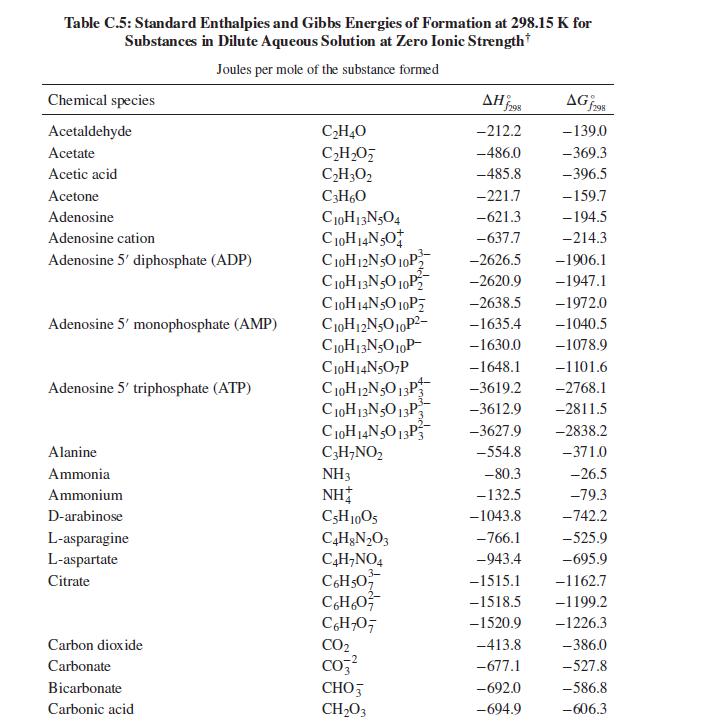
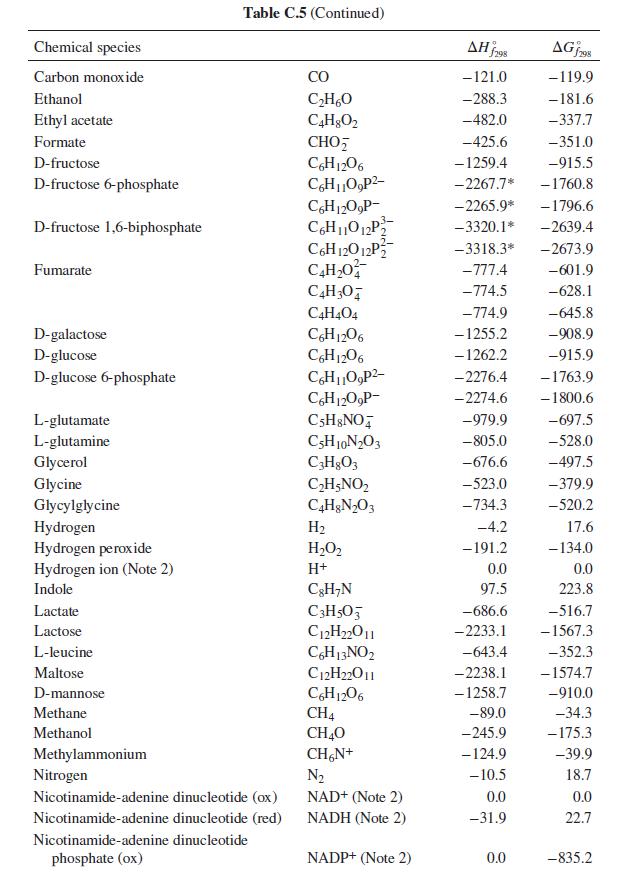
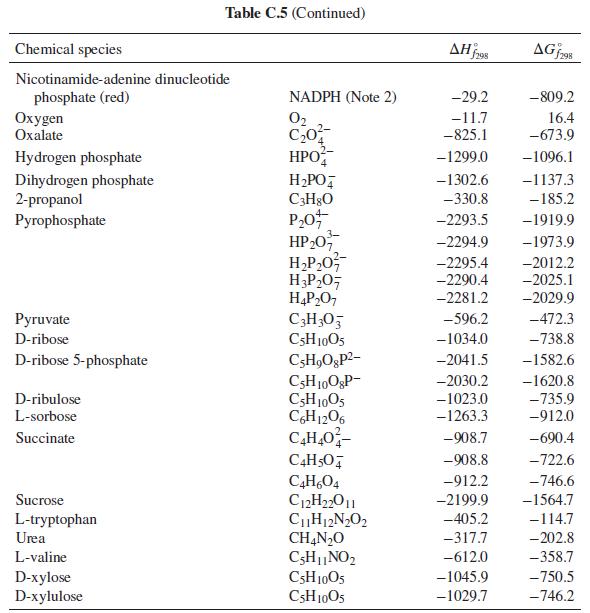
What is the heat effect of this reaction upon metabolizing 10 g of ethanol from a typical cocktail? What is the total heat effect for complete metabolism of the 10 g of ethanol to CO2 and water? How, if at all, is the perception of warmth that accompanies ethanol consumption related to these heat effects? For computing heat effects, you may neglect the temperature, pH, and ionic strength dependence of the enthalpy of reaction (i.e. apply the enthalpies of formation from Table C.5 of App. C at physiological conditions).
Table C.5 of App. C
Step by Step Answer:

Introduction To Chemical Engineering Thermodynamics
ISBN: 9781259696527
8th Edition
Authors: J.M. Smith, Hendrick Van Ness, Michael Abbott, Mark Swihart





