Consider a mixture of species 1, 2, and 3. The following equation of state is available for
Question:
Consider a mixture of species 1, 2, and 3. The following equation of state is available for the vapor phase:![]()
where,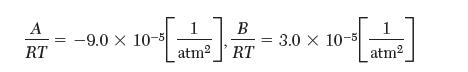
and y1, y2, and y3 are the mole fractions of species 1, 2 and 3, respectively. Consider a vapor mixture with 1 mole of species 1, 2 moles of species 2, and 2 moles of species 3 at a pressure of 50 atm and a temperature of 500 K. Calculate the following quantities: v, V, v1, v2, v3, V1.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





