Find the eutectic point for a binary mixture of cadmium and lead. You may assume that the
Question:
Find the eutectic point for a binary mixture of cadmium and lead. You may assume that the solid phases of these metals are completely immiscible and the liquid is completely miscible.
The following data are available for the pure species: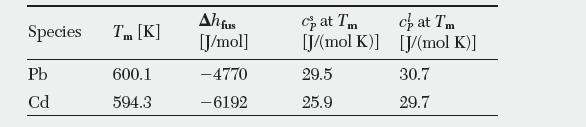
Perform calculations for the following cases:
(a) The liquid forms an ideal solution
(b) The liquid nonideality can be described by the two-suffi x Margules equation with A = 8,200 J/mol.
Compare with the experimentally determined eutectic at xPb = 0.719 and T = 521 K.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Question Posted:





