Assuming the reaction conditions (temperature, concentrations, and so on) are the same, compare the forward-direction rates for
Question:
Assuming the reaction conditions (temperature, concentrations, and so on) are the same, compare the forward-direction rates for reactions A and B in Problem 13.100.
Data from Problem 13-100
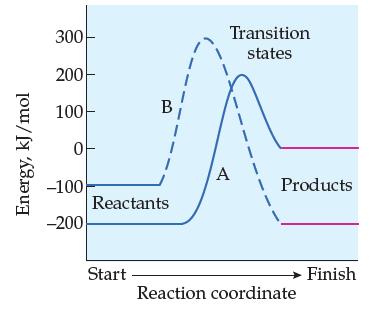
From the following reaction-energy profiles, determine whether reactions A and B are exothermic or endothermic in the forward direction:
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





