The mechanism for the endothermic reaction (a) Draw the reaction-energy profile for this reaction and label reactants,
Question:
The mechanism for the endothermic reaction
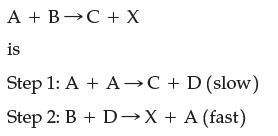
(a) Draw the reaction-energy profile for this reaction and label reactants, products, reaction intermediates, transition states, activation energies, and ΔErxn.
(b) What is the rate law for this reaction?
(c) If you wanted to quadruple the rate of this reaction, by what factor would you have to increase the concentration of A?
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:




