Consider molecules of the type H 2 A, where A is a group VIA atom (O, S,
Question:
Consider molecules of the type H2A, where A is a group VIA atom (O, S, Se, Te). Their relative boiling points are shown below.
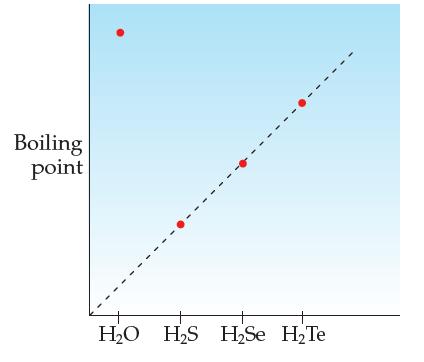
(a) Why is the boiling point trend H2S 2Se 2Te?
(b) The boiling point of H2O is well above the line that the others are on. Explain why this is so.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





