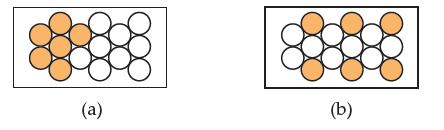
Consider the following two pieces of metal. Both consist of gold atoms (filled spheres) and silver atoms
Question:
Consider the following two pieces of metal. Both consist of gold atoms (filled spheres) and silver atoms (open spheres). Which would be considered heterogeneous? Explain why.
Transcribed Image Text:
(a) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
a Piece of metal a would be considered heterogeneous because it is made up of gold atoms and silver ...View the full answer

Answered By

Rohith Bellamkonda
I am studying in IIT Indore,the most prestigious institute of India.I love solving maths and enjoy coding
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Consider the following two risky asset worlds: Answer the following questions: a. Explain why the risk of any portfolio composed of (S) and (B) is less than weighted average risk of the two...
-
Dunkin Donuts What are all of Dunkin Donuts brands or product lines in this product mix? How does Dunkin Donuts promote itself? Starbucks What are all of Starbuck brands or product lines in this...
-
Consider the following two separate firms. One firm manufactures flexible packaging films for the snack, bakery, confectionery, and tobacco industries. Its manufacturing process has been quite stable...
-
For the curve defined by F(t) = (etcos(t), e* sin(t)) find the unit tangent vector, unit normal vector, normal acceleration, and tangential acceleration at 5A t 6 T N (5r) = 6 5T 6 aN ||
-
(a) What is the basic difference between the cost method and the par value method of accounting for treasury stock? (b) How will total stockholders equity differ, if at all, under the two methods?
-
For the cash flows shown, calculate the future worth in year 8 using i = 10% peryear. Year Cash Flow, 100 00 100 100 100 300 300
-
Jason Cannon purchased a defective tractor for which Cannon brought numerous causes of action against Bodensteiner Implement Company (Bodensteiner). One of the claims was breach of express...
-
Following are preacquisition financial balances for Padre Company and Sol Company as of December 31. Also included are fair values for Sol Company accounts. On December 31, Padre acquires Sols...
-
The figure shows a schematic diagram of a simple mass spectrometer, consisting of a velocity selector and a particle detector and being used to separate singly ionized atoms (q+e 1.602e-19 C) of gold...
-
Beaker (a) contains the pure compound called hydrogen peroxide (formula H 2 O 2 ). Beaker (b) contains the pure compound called water (H 2 O). Both compounds are made to undergo a change. Which...
-
Which one of the following is a compound: ozone, 18-karat gold (made by melting gold and other metals together), NaCl, liquid nitrogen, iced tea?
-
Show that the structure function for a three-component system that functions if and only if component 1 functions and at least one of components 2 or 3 functions is given by $(X,X2X;) = X, max(X2,...
-
Shi Import-Export's balance sheet shows $400 million in debt, $60 million in preferred stock, and $260 million in total common equity. Shi's tax rate is 15%, rd = 9%, rps = 8.9%, and rs = 13%. If Shi...
-
A firm has sales of $1.3 million, and 15 percent of the sales are for cash. The year-end accounts receivable balance is $150,000. What is the average collection period? (Use a 360-day year. Do not...
-
If the 1-year rate of return is 20% and the interest rates are constant, what is the 5-year holding rate of return? Q 2-11 If you invest $2,000 today and it earns 25% per year, how much will you have...
-
Village Finance Co . advanced three loans to Kamiko $ 3 , 1 0 0 on June 9 , $ 2 , 7 0 0 on August 1 4 , and $ 2 , 2 0 0 on October 1 8 . Simple interest at 7 . 7 0 % was charged on all three loans,...
-
A company has issued preferred stock with an annual dividend of $2.42 that will be paid in perpetuity. The current price of the stock is $24.6. What is the expected rate of return on the preferred...
-
Player Corporation makes an equity investment costing $73,000 and classifies it as non-trading. At December 31, the fair value of the investment is $67,000. Instructions Prepare the adjusting entry...
-
Wal-Mart is the second largest retailer in the world. The data file on the disk holds monthly data on Wal-Marts revenue, along with several possibly related economic variables. a) Using computer...
-
Identify the reagents necessary to achieve each of the following transformations: Br Br - " Br Br "Br
-
Determine the configuration for every chirality center in each of the following compounds. a. b. c. - - - - - - CH- - CH- II
-
For each of the following reactions predict the sign of G. If a prediction is not possible because the sign of G will be temperature dependent, describe how G will be affected by raising the...
-
Company Jetstar: a. Define the core product: what is/are the core benefits and features of the chosen service product? Who are the customers for this core service product (i.e., their pains and...
-
How can future value help assess the worth of employee benefits? Explain.
-
if a company produces more product than they planned in their budget, will that impact the overhead amount in terms of under or over applied overhead?

Study smarter with the SolutionInn App


