Draw a Bohr diagram for the highest-energy excited state you can make using the three shells shown
Question:
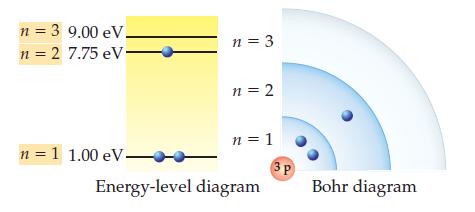
Draw a Bohr diagram for the highest-energy excited state you can make using the three shells shown in Practice Problem 4.9 and exciting only a single electron.
Data from Problem 4.9
The ground state for the lithium (Li) atom and the scaled energies of its shells are shown below. Draw a Bohr diagram for the lowest-energy excited state of lithium.
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:




