Draw three beakers as shown in WorkPatch 15.3, but change the labels to make them reflect the
Question:
Draw three beakers as shown in WorkPatch 15.3, but change the labels to make them reflect the reaction mentioned in Practice Problem 15.9. Then list all species present in each beaker.
Data from Problem 15.9
How many moles of Ba(OH)2 would it take to neutralize 0.1 mole of hydrochloric acid?
Data from Workpatch 15.3
What species are present in each beaker?
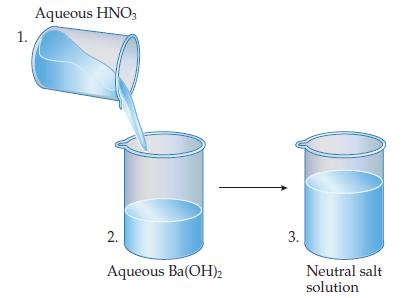
Ionic compounds are often called salts. What are the chemical formula and name of the salt that gives rise to the salt solution in beaker 3?
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:




