Draw three-beaker sets similar to those in WorkPatch 15.3 for Problems 15.91 and 15.92. Fill in the
Question:
Draw three-beaker sets similar to those in WorkPatch 15.3 for Problems 15.91 and 15.92. Fill in the species present in each beaker. Assume the H2SO4 dissociates completely to give two H3O+ ions (although it actually doesn’t do this).
Workpatch 15.3
What species are present in each beaker?
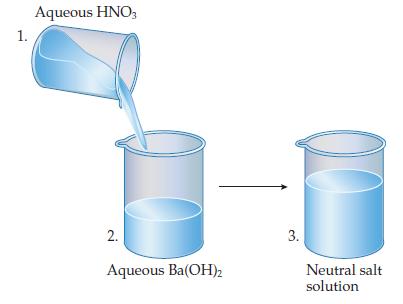
Ionic compounds are often called salts. What are the chemical formula and name of the salt that gives rise to the salt solution in beaker 3?
Data from Problem 15.91
How many moles of Ba(OH)2 does it take to neutralize 0.50 mole of H2SO4? Why does the resulting solution after the neutralization no longer conduct electricity? Why does the solution then conduct electricity after an excess of Ba(OH)2 is added?
Data from Problem 15.92
How many moles of Ba(OH)2 does it take to neutralize 2.5 moles of HNO3?
Step by Step Answer:

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver




