Indicate with an arrow the direction of the equilibrium shift and predict what will happen to the
Question:
Indicate with an arrow the direction of the equilibrium shift and predict what will happen to the amount of Fe2O3 (increases, decreases, unchanged, need more information) when the following stresses are applied to the following exothermic reaction:
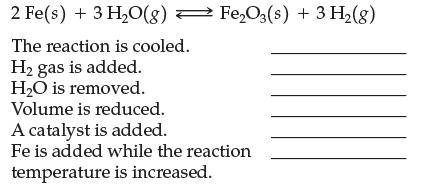
Transcribed Image Text:
2 Fe(s) + 3 H₂O(g)Fe₂O3(s) + 3 H₂(g) The reaction is cooled. H₂ gas is added. H₂O is removed. Volume is reduced. A catalyst is added. Fe is added while the reaction temperature is increased.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
Direction of equilibrium shift Cooling The reaction is exothermic so cooling the system will shift t...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Predict what will happen to the equilibrium price and quantity of oranges if the following events take place. (L03) a. A study finds that a daily glass of orange juice reduces the risk of heart...
-
Predict what will happen to aggregate output and the price level if the Federal Reserve increases the money supply at the same time that Congress implements and income tax cut.
-
Predict what will happen to interest rates if prices in the bond market become more volatile.
-
Consider the nutrition problem in Example 1 of Section 3.3. Solve the problem by the simplex method, and then determine the optimal quantities of soybeans and rice in the diet, and the new cost, if...
-
Determining Financial Statement Effects of Sales and Cost of Goods Sold and Issuance of Par Value Stock Using the following categories, indicate the effects of the following transactions. Use + for...
-
The managers of a chemical manufacturing plant want to determine whether recent safety training workshops have reduced the weekly number of reported safety violations at the facility. The management...
-
Assuming the same data as given in problem 9, was the well in each case profitable? Discuss your answer. Problem 9:- Property cost (acquisition cost). Drilling cost (one well). Estimated completion...
-
The Lucky Seven Company is an international clothing manufacturer. Its Redmond plant will become idle on December 31, 2014. Peter Laney, the corporate controller, has been asked to look at three...
-
Explain the ways in which the firms and industries that led cities' recoveries from de-industrialization are similar and different from the firms and industries that led the industrial revolution....
-
How does decreasing the temperature affect the value of K eq for an exothermic reaction? (a) Increases K eq (b) Decreases K eq (c) Does not change K eq
-
Shown below is a concentration versus time plot for the reaction A B. Using the data on the graph, determine the equilibrium constant for the reaction. Concentration (M)- 0.100 0.080 0.060 0.040...
-
Phillips Company manufactures air-conditioning units for commercial buildings and has noticed considerable variation in shipping expenses from month to month as per the data below: What is the fixed...
-
Why do some countries require transit visas? If the purpose is so unimportant.explain
-
Ed and Bonnie are married and have filed joint federal income tax returns for several years. Unfortunately, in 2020 Bonnie left Ed and Ed has not heard from her since her departure. In the meantime,...
-
4. For the following variable declaration and initialization, 1 char c = 'E'; What numerical value does the variable c have? (Give your answer as a decimal integer.)
-
An automobile has a maximum amplitude of 5 cm and a measured maximum acceleration of 2000 cm/s. Assuming that the automobile can be modeled as a single-degree- of-freedom system, calculate the...
-
On Nov. 1. 2022, Client prepays Eel Corp. for 24 months* worth of consulting services at $10,179/month (total payment was $10,179 x 24 months). The services begin on Nov. 1, 2022 and will be provided...
-
1) Using the financial information provided for Bliss Limited, prepare a cash flow statement, in a form suitable for publication, for the year ended 31 December 2011. 2) Using between 200 and 250...
-
The following information is available for Partin Company: Sales $598,000 Sales Returns and Allowances 20,000 Cost of Goods Sold 398,000 Selling Expense 69,000 Administrative Expense 25,000 Interest...
-
A bicycle is moving initially with a constant velocity along a level road. The bicyclist then decides to slow down, so she applies her brakes over a period of several seconds. Thereafter, she again...
-
Make a qualitative sketch of the position y as a function of time for the center of a yo-yo (the point at the middle of the axle). Also make sketches of the velocity and acceleration as functions of...
-
In SI units, velocity is measured in units of meters per second (m/s). Which of the following combinations of units can also be used to measure velocity? (a) Cm/s (b) Cm/s 2 (c) m 3 /(mm 2 s 2 ) (d)...
-
Describe Are you an applicant who is actively involved in the school's community?
-
Match the following, discussing the negotiation process: Column A 1 .Use your power to get the other party to give you what you want. 2. Make the first offer concession. 3. Start by asking for their...
-
Should we reorder? the Marks & Spencer approach A special case of the How much to order? decision in inventory control is the Should we order any more at all? decision. Retailers especially need...

Study smarter with the SolutionInn App


