Redraw the shells of Li, Be, B, and C shown in the above drawing to make the
Question:
Redraw the shells of Li, Be, B, and C shown in the above drawing to make the overall size of each atom fit the size trend. In each nucleus, write the total number of protons present. Which atom would be most difficult to ionize?
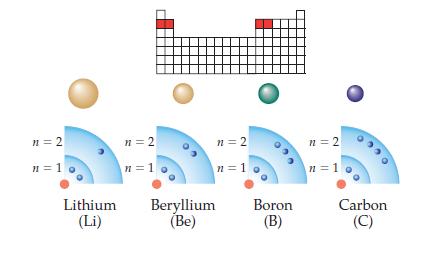
Transcribed Image Text:
n = 2 n=1 Lithium (Li) n=2 n=1 n = 2 n=1 Beryllium (Be) Boron (B) n=2 n=1 Carbon (C)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
Carbon would be the hardest to ionize because its shells are cl...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Read the case study "Southwest Airlines," found in Part 2 of your textbook. Review the "Guide to Case Analysis" found on pp. CA1 - CA11 of your textbook. (This guide follows the last case in the...
-
Read the case study and answer the question below with a one page response. What does a SWOT analysis reveal about the overall attractiveness of Under Armours situation? Founded in 1996 by former...
-
11) log, 16 4X220 4 ky 13) log, 8+log, (1-4x)=log, 63 15) log, 3x-log, 5=4 12) Evaluate 2 log, 7776-log, 729 14) log, (x-6)+log, (x-5)=1 loge (x-6)+ log 6 (x-5' 16) log 9+ logx = 4
-
What is the major difference between the disposition of units transferred out of a first department in a single-department factory versus a multiple-department factory?
-
Marlow Company acquired 40 percent of the voting shares of Brown Company on January 1, 20X8, for $85,000. The following results are reported for Brown Company: Required Give all journal entries...
-
What is the plain-meaning rule?
-
Journalize the following transactions that occurred in September 2016 for Cardinal. No explanations are needed. Identify each accounts payable and accounts receivable with the vendor or customer...
-
How to create a relational model from DDL statements and how you save a relational model design.?
-
Why is an electron in a shell that has a low value of n in a more stable arrangement than one in a shell that has a higher value of n?
-
The following statement(s) is true about the periodic table: (a) Elements in the same group have similar valence-shell electron configurations. (b) Elements in the same period have the same value of...
-
For y = log 10 x, if y increases by 2, then (choose the correct answer): (a) x increases by 20 (b) x decreases by 20 (c) x increases by a factor of 2 (d) x increases by a factor of 100
-
ABC Company and XYZ Company need to raise funds to pay for capital improvements at their manufacturing plants. ABC Company is a well-established firm with an excellent credit rating in the debt...
-
Jennifer McAfee recently received her university Masters degree and has decided to enter the mortgage brokerage business. Rather than work for someone else, she has decided to open her own shop. Her...
-
SlapShot plc has a fixed cost associated with buying and selling marketable securities of 100. The interest rate is currently 0.021 per cent per day, and the firm has estimated that the standard...
-
Saint-Michel SA needs a total of 54,000 in cash during the year for transactions and other purposes. Whenever cash runs low, it sells off 20,000 in securities and transfers the cash in. The interest...
-
TByrne Ltd is currently holding 700,000 in cash. It projects that over the next year its cash outflows will exceed cash inflows by 360,000 per month. How much of the current cash holding should be...
-
Some proponents of TQM assert that quality is free, that is, that quality is a never-ending quest and that improving product/service quality will reduce a firms total spending on quality. Others...
-
Discuss whether responsible human resources management should apply different standards for the home company and suppliers, for developed countries and developing countries, and for large companies...
-
The ground electronic state of O 2 is 3 g and the next two highest energy states are 1 g (7918 cm 1 ) and 1 + g (13195 cm 1 ), where the value in parentheses is the energy of the state relative...
-
Determine if the following transitions are allowed or forbidden: a. 3 u 3 g b. 1 + g 1 g c. 3 g 3 g d. 1 g 1 u
-
In a simple model used to analyze UV photoelectron spectra, the orbital energies of the neutral molecule and the cation formed by ejection of an electron are assumed to be the same. In fact, some...
-
https://youtu.be/Ks-_Mh1QhMc?si=gEKm-G-DhfjAF5Xz What exactly are we doing here? You will be summarizing the video posted on the link I left https://youtu.be/Ks- Mh1QhMc?si=3GslYP99K8ottAoe A summary...
-
Q1: Consider the sample data below. What are the functional dependencies you can identify? Exclude any dependencies you think are unlikely to hold given a large sample. Order Date (A) (B) Customer...
-
Consider the code shown below depicting f(x). What is (g(x)), (g(x)), and O(g(x)) for the function shown? Explain why. (5 pts) 1 12 2 3 4 5 for (int i = 0; i < n; i++) { for (int j = n; j > 0; j--) {...

Study smarter with the SolutionInn App


