Suppose the first step in the reaction of Problem 13.155 was the rate-determining step. Would the rate
Question:
Suppose the first step in the reaction of Problem 13.155 was the rate-determining step. Would the rate law be k[Cl2][CHCl3], k[Cl2], k[CHCl3], or k[Cl2]2? Explain.
Data from Problem 13.155
Write the overall balanced chemical equation that goes along with the mechanism:
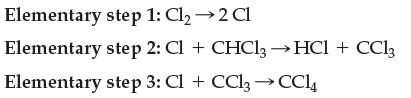
Transcribed Image Text:
Elementary step 1: Cl₂ → 2 Cl Elementary step 2: Cl + CHCl3→ HCl + CC13 Elementary step 3: Cl + CCl₂ → CC14
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
If the first step in the reaction of Problem 13155 was the ratedetermining step the rate law woul...View the full answer

Answered By

Ankur Gupta
I have a degree in finance from a well-renowned university and I have been working in the financial industry for over 10 years now. I have a lot of experience in financial management, and I have been teaching financial management courses at the university level for the past 5 years. I am extremely passionate about helping students learn and understand financial management, and I firmly believe that I have the necessary skills and knowledge to effectively tutor students in this subject.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
Write the overall balanced chemical equation that goes along with the mechanism: Elementary step 1: Cl 2 Cl Elementary step 2: Cl + CHCl3 HCl + CC13 Elementary step 3: Cl + CCl CC14
-
Suppose the experimental rate law for the reaction in Practice Problem 13.24 is: Rate = k[Y 2 ] 2 Use the step postulated in Practice Problem 13.24 as the first step in a possibly correct mechanism...
-
The object is to study the kinetics of the reaction between peroxodisulfate and iodide ions. The I 3 formed in reaction (a) is actually a complex of iodine, I 2 and iodide ion, I. Thiosulfate ion, S...
-
A ball, which we can treat as a point charge, has a charge of +Q. This ball is 50 cm away from a ball of charge-100, which is fixed in position. The +Q ball is 30 cm vertically below, and 40 cm...
-
Analyzing the Effects of Transactions in T-Accounts Granger Service Company, Inc., was organized by Ted Granger and five other investors. The following activities occurred during the year: a....
-
Axel Telecommunications has a target capital structure that consists of 70% debt and 30% equity. The company anticipates that its capital budget for the upcoming year will be $3,000,000. If Axel...
-
The efficiency of a light source is the percentage of its energy input that gets radiated as visible light. If some of the blue light in an LED is used to cause a fluorescent material to glow, A. The...
-
Sensitivity of EOQ to changes in relevant ordering and carrying costs, cost of prediction error. Alpha Companys annual demand for its only product, XT-590, is 10,000 units. Alpha is currently...
-
About Patagonia: What is the role of the digital channels of the brand Patagonia under investigation in creating a relevant user experience? Do they provide a great user experience to your opinion?...
-
The process to make ammonia (NH 3 ) gas is called the Haber process. The chemical reaction is shown below. To make it go fast enough tobe useful, it has to be run at high temperatures (400 C) and...
-
Suppose a postulated mechanism does generate the experimental rate law, and when the elementary steps are added together, the balanced equation for the overall reaction is generated. What can you say...
-
Why is it important to obtain secondary data before primary data?
-
Discuss and describe the types of water supplies for fire protection systems. . You might consider the following questions to help you get started Define friction loss and discuss how it affects...
-
A45kgchild on a sled is pulled through6.00mon a horizontal field of ice with minimal friction. A60Nforce is applied horizontally. If the child is initially at rest, how fast will he/she be going at...
-
What diameter of vertical tube would allow mayonnaise (= 1,200 kg/m3) to flow under its own weight?
-
Nick's utility over hummus (h) and books (b) is described by U(Qh, Qb) = Q0.7 h Q0.3 b . (a) Nick has $60 to spend. The price of hummus is $6 per unit, and the price of books is $18 per unit. Write...
-
1. Which force is directly acting on a car to accelerate/decelerate the car: the friction from the ground or the force from the engine? Explain your reasons. 2. To keep a car moving without slipping,...
-
Nick and Jolene are married. Nick is 61 and retired in 2010 from his job with Amalgamated Company. Jolene is 56 and works part-time as a special education teacher. Nick and Jolene have a substantial...
-
Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [HO]. Be sure to enter Keq in proper scientific notation. (a) ammonia (acting as a base) reacts...
-
Water at 10C is flowing at 0.075 m 3 /s. Calculate the weight flow rate and the mass flow rate.
-
Oil for a hydraulic system (sg = 0.90) is flowing at 2 .35 10 -3 m 3 /s . Calculate the weight flow rate and mass flow rate.
-
After the refrigerant from Problem 6.31 flashes into a vapor, its specific weight is 12.50 N/m 3 . If the weight flow rate remains at 28.5 N/h, compute the volume flow rate. In Problem A liquid...
-
Baillie Power leased high-tech electronic equipment from Courtney Leasing on January 1, 2024. Courtney purchased the equipment from Doane Machines at a cost of $260,000, its fair value. Note: Use...
-
(4.5) A visible photon with energy 2 eV is absorbed by a macroscopic body held at room temperature. By what factor does for the macroscopic body change? Repeat the calculation for a photon which...
-
A charge 4.98 nC is placed at the origin of an xy-coordinate system, and a charge -2.02 nC is placed on the positive x-axis at x = 4.04 cm. A third particle, of charge 6.05 nC is now placed at the...

Study smarter with the SolutionInn App


