The process to make ammonia (NH 3 ) gas is called the Haber process. The chemical reaction
Question:
The process to make ammonia (NH3) gas is called the Haber process. The chemical reaction is shown below. To make it go fast enough to be useful, it has to be run at high temperatures (400 °C) and over the surface of a catalyst.
![3H(8)+N(8) Keq 2 NH3(8) [NH3) [H][N] = 0.00016 (at 400C)](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1701/9/4/0/52465718d2cec50a1701940526224.jpg)
As you can see, the equilibrium lies far to the right, but chemical engineers have found a way to perform the reaction such that you can keep feeding in hydrogen and nitrogen gas and obtain pure ammonia. They take advantage of the following boiling point (bp) information and Le Châtelier’s principle.
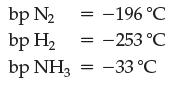
Design a reaction vessel to accomplish this.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:





