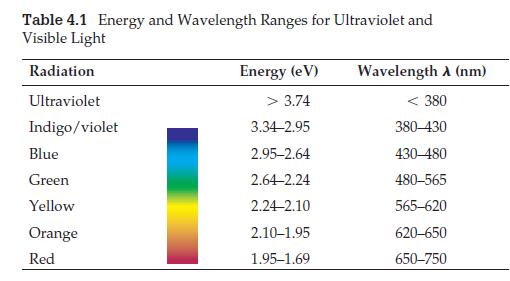
The hydrogen atom has three other visible lines in its line spectrum: green, blue, and indigo/violet. Use
Question:
The hydrogen atom has three other visible lines in its line spectrum: green, blue, and indigo/violet. Use the energy-level diagram for hydrogen and Table 4.1 to determine the electron jumps responsible for these lines.

Transcribed Image Text:
Indigo/ Blue violet Green Red n = 3n = 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Green n 4 to n 2 blue n 5 to n 2 indigoviolet n 6 to n ...View the full answer

Answered By

Mamba Dedan
I am a computer scientist specializing in database management, OS, networking, and software development. I have a knack for database work, Operating systems, networking, and programming, I can give you the best solution on this without any hesitation. I have a knack in software development with key skills in UML diagrams, storyboarding, code development, software testing and implementation on several platforms.
4.90+
49+ Reviews
119+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
A group of local behavior analysts gets together quarterly for a continuing education presentation followed by a group social and informal dinner. One of the members of our group is the owner of a...
-
Given the following energy level diagram for an atom that contains an electron in the n = 3 level, answer the following questions. a. Which transition of the electron will emit light of the lowest...
-
(a) Suppose an unknown element has a absorption spectrum with lines at 2.5, 4, and 5.1eV above its ground state and an ionization energy of 11.5eV. Draw an energy level diagram for this element. (b)...
-
The Kc for the following reaction is 9.30 X 10^-2 at 25C:PCl5(g) <-> PCl3(g) + Cl2(g) How many moles & grams of PCl5 must be added to a 2-literflask to obtain a Cl2 concentration of 0.150M...
-
What is meant by the term equivalent production as used in the process cost system?
-
Ambrose Corporation owns 75 percent of Kroop Company's common stock, acquired at underlying book value on January 1, 20X4. At the acquisition date, the book values and fair values of Kroop's assets...
-
Rockstar Games, a subsidiary of Take-Two Interactive, released the video game Grand Theft Auto V in 2013. The game features a character named Lacey Jonas, a self-proclaimed actress slash singer and...
-
Superior Markets, Inc., operates three stores in a large metropolitan area. A segmented absorption costing income statement for the company for the last quarter is given below: The North Store has...
-
Cash Receipt Schemes and Other Asset Misappropriations, identify and describe two big data and data analytic techniques each for detecting skimming, cash larceny, and noncash misappropriations.
-
Using dots to represent potassiums 19 electrons, fill the subshells in the diagram below to arrive at the ground-state energy- level diagram for the potassium atom. Select the diagram that gives an...
-
What is wrong with this Bohr model of the beryllium (Be) atom (atomic number 4)? n = 3 n = 2 n = 1 4 p
-
To best assess an ETFs performance, which reflects the impact of portfolio rebalancing expenses and other fees, an investor should: A. review daily return differences between the ETF and its...
-
An investor holds a portfolio of 3 securities. She invests 30 per cent in A, 30 per cent in B, and 40 per cent in C. The betas on A, B, and C are 1.5, 0.6 and 1.1 respectively. If E(R M ) = 12% and R...
-
Champions SA is considering a change in its cash-only sales policy. The new terms of sale would be net one month. Based on the following information, determine if Champions should proceed. Describe...
-
You plan to purchase a company and wish to estimate the expected return on the companys equity using a three-factor model. You believe the appropriate factors are the market return, the percentage...
-
You have recently been employed as a finance consultant to Bread plc. The CEO wants to use an APT model to estimate the required return on the companys stock, and the risk factors he plans to use in...
-
The market portfolio has an expected return of 12 per cent and a standard deviation of 10 per cent. The risk-free rate is 5 per cent. (a) What is the expected return on a well-diversified portfolio...
-
Through its accreditation function, AACSB International (www.aacsb.edu) seeks continuous quality improvement in the content, delivery, and administration of management education. The AACSB in fact...
-
Why is disclosure of depreciation or amortization methods and rates so important?
-
Evaluate the difference between change in energy at 0 K in the absence of zero point vibration and both change in enthalpy and in free energy for real molecules at 298 K. Consider both a...
-
Hydrazine would be expected to adopt a conformation in which the N~H bonds stagger. There are two likely candidates, one with the lone pairs on nitrogen anti to each other and the other with the lone...
-
DielsAlder cycloaddition of 1, 3-butadiene with acrylonitrile requires that the diene be in a cis (or cis-like) conformation: In fact, the diene exists primarily in a trans conformation, the cis...
-
What, roughly, is the percent uncertainty in the volume of a spherical beach ball whose radius is r = 2.70 0.07 m? Express your answer using one significant figure.
-
Describe how the analysis process training can be focused on business priorities and critical strategies essential to the organization's success. Discuss the factors that hinder and facilitate...
-
Design a comprehensive communication strategy for a multinational corporation (MNC) that aims to rebrand its image and reposition itself as a socially responsible and environmentally conscious...

Study smarter with the SolutionInn App


