What is the mass in grams of sucrose necessary to saturate 100.0 lb of water at 90
Question:
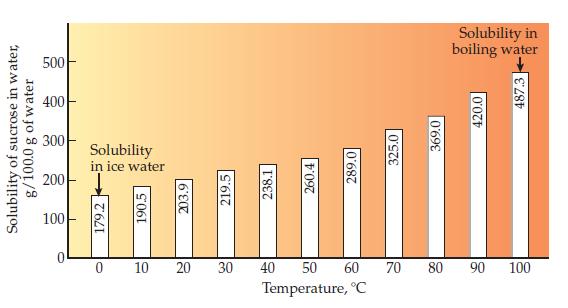
What is the mass in grams of sucrose necessary to saturate 100.0 lb of water at 90 °C? (Use the top graph on page 462; 1 lb = 453.6 g.)
Top Graph from page 462
Transcribed Image Text:
Solubility of sucrose in water, g/100.0 g of water 500 400 300 200 100 T Solubility in ice water 179.25 0 190.5 203.9 10 20 219.5 30 238.1 260.4 40 289.0 50 60 Temperature, °C 325.0 70 369.0 80 Solubility in boiling water 420.0 487.3 90 100
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (5 reviews)
To find the mass of sucrose necessary to saturate 100...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Introductory Chemistry Atoms First
ISBN: 9780321927118
5th Edition
Authors: Steve Russo And Michael Silver
Question Posted:
Students also viewed these Sciences questions
-
What is the mass in grams of 1 molecule of H2O?
-
What is the mass in grams of 1 atom of Al?
-
What is the mass in grams of solute in? (a) 250 mL of 0.264 M H2O2 (b) 37.0 mL of 5.75 x 10-4 M benzoic acid (122 g / mol)
-
Read the article Somatoform and Related Disorders: An Update which address personality disorders, substance abuse, as any of these behaviors are often considered to be predictors of crime and/or...
-
Joan owns a print shop and has a difficult time figuring out what to bid on jobs. She knows how to estimate the cost of materials and labor on different jobs but is confused about how to handle many...
-
Survey the literature from the past six months to find one application each of DSS, BI, and analytics. Summarize the applications on one page and submit it with the exact sources.
-
The enzyme lipase catalyzes the hydrolysis of esters of fatty acids. The hydrolysis of p-nitrophenyloctanoate was followed by measuring the appearance of p-nitrophenol in the reaction mixture: The...
-
Terry Wade, the new controller of Hellickson Company, has reviewed the expected useful lives and salvage values of selected depreciable assets at the beginning of 2014. His findings are as follows....
-
1C.1 What's the largest gravitational force you can produce between the two masses? (Note: it's convenient to express the forces in this sim in terms of piconewtons-pN. One piconewton is 0.000000 000...
-
(a) Use these data to plot solubility as a function of temperature for KCl and Li 2 SO 4 : (b) Using the plot, estimate the solubility of both compounds in water at 70C. (c) How much of each compound...
-
Aquatic life is often damaged when hot water is discharged from power stations into rivers and lakes. What might this have to do with gas solubility in water?
-
The data below is taken from the BOP of Switzerland. Based on this data, decide whether the following statement is true or false and explain your answer. "From 1979 to 1982, foreigners have been net...
-
Savor the Sweet Bakery has been selling 550 boxes of cupcakes per month at a price of $19/box. When they raised their price to $21/box, they sold only 450 boxes. 1)What is the price elasticity of...
-
How does the immune system distinguish between self and non-self antigens, and what are the consequences of autoimmune diseases where this distinction breaks down?
-
In footnotes to its 2016 annual report, Bancfirst Corp. reported that held-to-maturity debt securities with an amortized cost of $4,365 thousand had an estimated fair value of $4,403 thousand. a....
-
Bobby has a real salary of $61,430. Julianne has a real salary of $124,875. Neither one contributes to a retirement plan. a. If Bobby & Julianne are married and take a standard deduction, how much...
-
On January 1, 2018, the Coldstone Corporation adopted the dollar-value LIFO retail inventory method. Beginning inventory at cost and at retail were $170,000 and $273,000, respectively. Net purchases...
-
Compute the mean for the followingnumbers. 17.3 44.5 31.6 40.0 52.8 38.8 30. 78.5
-
Record the following selected transactions for March in a two-column journal, identifying each entry by letter: (a) Received $10,000 from Shirley Knowles, owner. (b) Purchased equipment for $35,000,...
-
Find the pH of a 0.500 M NaCN solution. The salt dissociates completely into Na + and CN - , and the Na + has no acid or base properties.
-
A 1.0 L buffer solution contains 0.15 mol acetic acid and 0.10 mol sodium acetate. The K a for acetic acid is 1.8 10 -5 . Calculate the pH of the solution before and after a 0.010 mol sample of NaOH...
-
Calculate the molar solubility of iron carbonate FeCO 3 in pure water.
-
(a). Show that the eigenvalue problem for the Gauss-Seidel iteration matrix may be expressed in the form RGSW = Aw Uw (DL)w, = where D, -L, -U is the usual diagonal lower triangular and upper...
-
The use of social media has increased day by day and businesses are finding it as a very helpful tool to grow and earn profit. One such coffee company is Second cup Canada a coffee company like star...
-
If it is industrial good identify buying situations and buying centers. Business Brief Tajheez as previously introduced in Week 1, is a reliable online app-based valuable service for homeowners and...

Study smarter with the SolutionInn App


