In the Ostwald process, ammonia and oxygen gases combine to give nitrogen monoxide gas and steam using
Question:
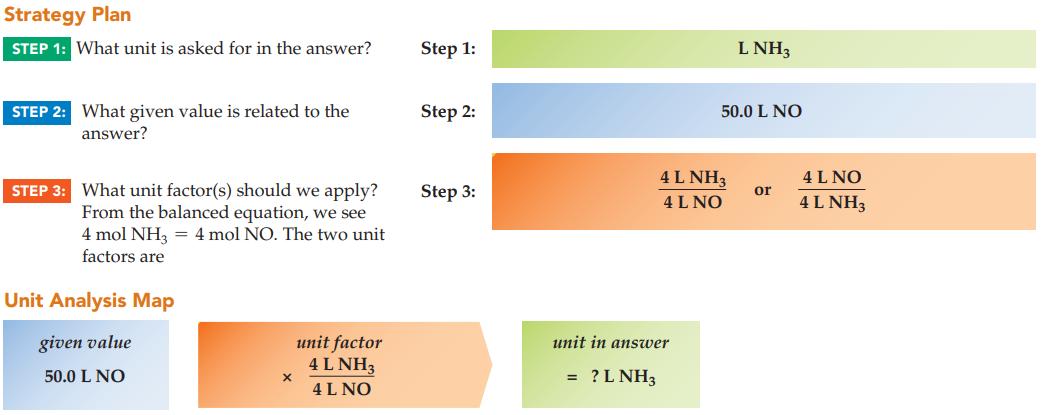
In the Ostwald process, ammonia and oxygen gases combine to give nitrogen monoxide gas and steam using a platinum catalyst. The NO is then converted to nitric acid, HNO3. If 50.0 L of NO are produced, what is the volume of NH3 required? Assume all gaseous volumes are measured at the same conditions of temperature and pressure.
![]()
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Introductory Chemistry Concepts And Critical Thinking
ISBN: 9780321804907
7th Edition
Authors: Charles Corwin
Question Posted:





